The abundance of Met30p limits SCF(Met30p) complex activity and is regulated by methionine availability
- PMID: 11027256
- PMCID: PMC86396
- DOI: 10.1128/MCB.20.21.7845-7852.2000
The abundance of Met30p limits SCF(Met30p) complex activity and is regulated by methionine availability
Abstract
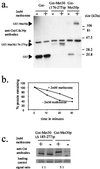
Ubiquitin-mediated degradation plays a crucial role in many fundamental biological pathways, including the mediation of cellular responses to changes in environmental conditions. A family of ubiquitin ligase complexes, called SCF complexes, found throughout eukaryotes, is involved in a variety of biological pathways. In Saccharomyces cerevisiae, an SCF complex contains a common set of components, namely, Cdc53p, Skp1p, and Hrt1p. Substrate specificity is defined by a variable component called an F-box protein. The F- box is a approximately 40-amino-acid motif that allows the F-box protein to bind Skp1p. Each SCF complex recognizes different substrates according to which F-box protein is associated with the complex. In yeasts, three SCF complexes have been demonstrated to associate with the ubiquitin-conjugating enzyme Cdc34p and have ubiquitin ligase activity. F-box proteins are not abundant and are unstable. As part of the SCF(Met30p) complex, the F-box protein Met30p represses methionine biosynthetic gene expression when availability of L-methionine is high. Here we demonstrate that in vivo SCF(Met30p) complex activity can be regulated by the abundance of Met30p. Furthermore, we provide evidence that Met30p abundance is regulated by the availability of L-methionine. We propose that the cellular responses mediated by an SCF complex are directly regulated by environmental conditions through the control of F-box protein stability.
Figures



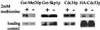

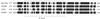
Similar articles
-
Overproduction of polypeptides corresponding to the amino terminus of the F-box proteins Cdc4p and Met30p inhibits ubiquitin ligase activities of their SCF complexes.Eukaryot Cell. 2003 Feb;2(1):123-33. doi: 10.1128/EC.2.1.123-133.2003. Eukaryot Cell. 2003. PMID: 12582129 Free PMC article.
-
Identification of residues in the WD-40 repeat motif of the F-box protein Met30p required for interaction with its substrate Met4p.Mol Genet Genomics. 2005 Jun;273(5):361-70. doi: 10.1007/s00438-005-1137-6. Epub 2005 May 10. Mol Genet Genomics. 2005. PMID: 15883825
-
The amino-terminal portion of the F-box protein Met30p mediates its nuclear import and assimilation into an SCF complex.J Biol Chem. 2004 Feb 20;279(8):6674-82. doi: 10.1074/jbc.M308875200. Epub 2003 Dec 5. J Biol Chem. 2004. PMID: 14660673
-
Proteolysis and the G1-S transition: the SCF connection.Curr Opin Genet Dev. 1998 Feb;8(1):36-42. doi: 10.1016/s0959-437x(98)80059-2. Curr Opin Genet Dev. 1998. PMID: 9529603 Review.
-
The F-box: a new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction.Prog Biophys Mol Biol. 1999;72(3):299-328. doi: 10.1016/s0079-6107(99)00010-3. Prog Biophys Mol Biol. 1999. PMID: 10581972 Review.
Cited by
-
Repression of sulfate assimilation is an adaptive response of yeast to the oxidative stress of zinc deficiency.J Biol Chem. 2009 Oct 2;284(40):27544-56. doi: 10.1074/jbc.M109.042036. Epub 2009 Aug 5. J Biol Chem. 2009. PMID: 19656949 Free PMC article.
-
Overproduction of polypeptides corresponding to the amino terminus of the F-box proteins Cdc4p and Met30p inhibits ubiquitin ligase activities of their SCF complexes.Eukaryot Cell. 2003 Feb;2(1):123-33. doi: 10.1128/EC.2.1.123-133.2003. Eukaryot Cell. 2003. PMID: 12582129 Free PMC article.
-
Activation of the S-phase checkpoint inhibits degradation of the F-box protein Dia2.Mol Cell Biol. 2010 Jan;30(1):160-71. doi: 10.1128/MCB.00612-09. Mol Cell Biol. 2010. PMID: 19858292 Free PMC article.
-
Multi-omics characterization of the necrotrophic mycoparasite Saccharomycopsis schoenii.PLoS Pathog. 2019 May 9;15(5):e1007692. doi: 10.1371/journal.ppat.1007692. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31071195 Free PMC article.
-
Downregulation of autophagy by Met30-mediated Atg9 ubiquitination.Proc Natl Acad Sci U S A. 2021 Jan 5;118(1):e2005539118. doi: 10.1073/pnas.2005539118. Proc Natl Acad Sci U S A. 2021. PMID: 33443148 Free PMC article.
References
-
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper J W, Elledge S J. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. - PubMed
-
- Barral Y, Jentsch S, Mann C. G1 cyclin turnover and nutrient uptake are controlled by a common pathway in yeast. Genes Dev. 1995;9:399–409. - PubMed
-
- Craig K L, Tyers M. The F-box: a new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction. Prog Biophys Mol Biol. 1999;72:299–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
