Changes in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomy
- PMID: 11007885
- PMCID: PMC6772759
- DOI: 10.1523/JNEUROSCI.20-19-07279.2000
Changes in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomy
Abstract
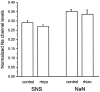
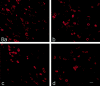
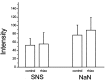
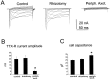
Two TTX-resistant sodium channels, SNS and NaN, are preferentially expressed in c-type dorsal root ganglion (DRG) neurons and have been shown recently to have distinct electrophysiological signatures, SNS producing a slowly inactivating and NaN producing a persistent sodium current with a relatively hyperpolarized voltage-dependence. An attenuation of SNS and NaN transcripts has been demonstrated in small DRG neurons after transection of the sciatic nerve. However, it is not known whether changes in the currents associated with SNS and NaN or in the expression of SNS and NaN channel protein occur after axotomy of the peripheral projections of DRG neurons or whether similar changes occur after transection of the central (dorsal root) projections of DRG neurons. Peripheral and central projections of L4/5 DRG neurons in adult rats were axotomized by transection of the sciatic nerve and the L4 and L5 dorsal roots, respectively. DRG neurons were examined using immunocytochemical and patch-clamp methods 9-12 d after sciatic nerve or dorsal root lesion. Levels of SNS and NaN protein in the two types of injuries were paralleled by their respective TTX-resistant currents. There was a significant decrease in SNS and NaN signal intensity in small DRG neurons after peripheral, but not central, axotomy compared with control neurons. Likewise, there was a significant reduction in slowly inactivating and persistent TTX-resistant currents in these neurons after peripheral, but not central, axotomy compared with control neurons. These results indicate that peripheral, but not central, axotomy results in a reduction in expression of functional SNS and NaN channels in c-type DRG neurons and suggest a basis for the altered electrical properties that are observed after peripheral nerve injury.
Figures

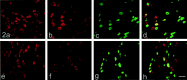

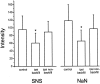
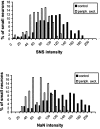
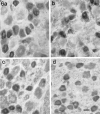


Similar articles
-
Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons.J Neurosci. 2000 Dec 1;20(23):8754-61. doi: 10.1523/JNEUROSCI.20-23-08754.2000. J Neurosci. 2000. PMID: 11102483 Free PMC article.
-
Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons.Brain Res Mol Brain Res. 1999 Apr 20;67(2):267-82. doi: 10.1016/s0169-328x(99)00070-4. Brain Res Mol Brain Res. 1999. PMID: 10216225
-
Rescue of alpha-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo.J Neurophysiol. 1998 May;79(5):2668-76. doi: 10.1152/jn.1998.79.5.2668. J Neurophysiol. 1998. PMID: 9582237
-
Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions.J Neurosci. 1998 Mar 15;18(6):2174-87. doi: 10.1523/JNEUROSCI.18-06-02174.1998. J Neurosci. 1998. PMID: 9482802 Free PMC article. Review.
-
NaN/Nav1.9: a sodium channel with unique properties.Trends Neurosci. 2002 May;25(5):253-9. doi: 10.1016/s0166-2236(02)02150-1. Trends Neurosci. 2002. PMID: 11972962 Review.
Cited by
-
Ion Channel and Transporter Involvement in Chemotherapy-Induced Peripheral Neurotoxicity.Int J Mol Sci. 2024 Jun 14;25(12):6552. doi: 10.3390/ijms25126552. Int J Mol Sci. 2024. PMID: 38928257 Free PMC article. Review.
-
Contactin associates with sodium channel Nav1.3 in native tissues and increases channel density at the cell surface.J Neurosci. 2004 Aug 18;24(33):7387-99. doi: 10.1523/JNEUROSCI.0322-04.2004. J Neurosci. 2004. PMID: 15317864 Free PMC article.
-
Voltage gated sodium channels as therapeutic targets for chronic pain.J Pain Res. 2019 Sep 9;12:2709-2722. doi: 10.2147/JPR.S207610. eCollection 2019. J Pain Res. 2019. PMID: 31564962 Free PMC article. Review.
-
Glycosylation alters steady-state inactivation of sodium channel Nav1.9/NaN in dorsal root ganglion neurons and is developmentally regulated.J Neurosci. 2001 Dec 15;21(24):9629-37. doi: 10.1523/JNEUROSCI.21-24-09629.2001. J Neurosci. 2001. PMID: 11739573 Free PMC article.
-
Sodium channels and pain: from toxins to therapies.Br J Pharmacol. 2018 Jun;175(12):2138-2157. doi: 10.1111/bph.13962. Epub 2017 Sep 2. Br J Pharmacol. 2018. PMID: 28749537 Free PMC article. Review.
References
-
- Acheson A, Lindsay RM. Non target-derived roles of the neurotrophins. Philos Trans R Soc Lond B Biol Sci. 1996;351:417–422. - PubMed
-
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Science. 1996;379:257–262. - PubMed
-
- Akopian AN, Soulova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. - PubMed
-
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
