Regulation of somatodendritic GABAA receptor channels in rat hippocampal neurons: evidence for a role of the small GTPase Rac1
- PMID: 10995817
- PMCID: PMC6772837
- DOI: 10.1523/JNEUROSCI.20-18-06743.2000
Regulation of somatodendritic GABAA receptor channels in rat hippocampal neurons: evidence for a role of the small GTPase Rac1
Abstract
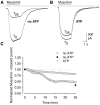
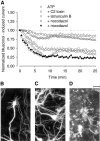
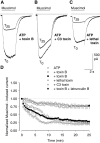
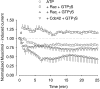
The role of the cytoskeleton in the activity of GABA(A) receptors was investigated in cultured hippocampal neurons. Receptor currents were measured with the whole-cell patch-clamp technique during repetitive stimulation with 1 microm muscimol. After destruction of the microtubular system with nocodazol, muscimol-induced currents showed a rundown by 78%. A similar rundown was observed when actin fibers were destroyed with latrunculin B or C2 toxin of Clostridium botulinum. Because the small GTPases of the Rho family RhoA, Rac1, and Cdc42 are known to control the organization of actin fibers, we investigated their possible involvement. Inactivation of the GTPases with clostridial toxins, as well as intracellular application of recombinant Rho GTPases, indicated that active Rac1 was necessary for full GABA(A) receptor activity. Immunocytochemical labeling of the receptors showed that the disappearance of receptor clusters in the somatic membrane as induced by muscimol stimulation was enhanced by Rac1 inactivation. It is suggested that Rac1 participates in the regulation of GABA(A) receptor clustering and/or recycling.
Figures

Similar articles
-
Rundown of somatodendritic N-methyl-D-aspartate (NMDA) receptor channels in rat hippocampal neurones: evidence for a role of the small GTPase RhoA.Br J Pharmacol. 1999 Jul;127(5):1060-3. doi: 10.1038/sj.bjp.0702643. Br J Pharmacol. 1999. PMID: 10455249 Free PMC article.
-
Chronic ethanol exposure alters the levels, assembly, and cellular organization of the actin cytoskeleton and microtubules in hippocampal neurons in primary culture.Toxicol Sci. 2010 Dec;118(2):602-12. doi: 10.1093/toxsci/kfq260. Epub 2010 Sep 9. Toxicol Sci. 2010. PMID: 20829428
-
Abl tyrosine kinase promotes dendrogenesis by inducing actin cytoskeletal rearrangements in cooperation with Rho family small GTPases in hippocampal neurons.J Neurosci. 2004 Sep 29;24(39):8510-21. doi: 10.1523/JNEUROSCI.1264-04.2004. J Neurosci. 2004. PMID: 15456825 Free PMC article.
-
Coordination between Rac1 and Rab Proteins: Functional Implications in Health and Disease.Cells. 2019 Apr 29;8(5):396. doi: 10.3390/cells8050396. Cells. 2019. PMID: 31035701 Free PMC article. Review.
-
Stress-Sensitive Protein Rac1 and Its Involvement in Neurodevelopmental Disorders.Neural Plast. 2020 Nov 24;2020:8894372. doi: 10.1155/2020/8894372. eCollection 2020. Neural Plast. 2020. PMID: 33299404 Free PMC article. Review.
Cited by
-
The cytotoxic necrotizing factors from Yersinia pseudotuberculosis and from Escherichia coli bind to different cellular receptors but take the same route to the cytosol.Infect Immun. 2007 Jul;75(7):3344-53. doi: 10.1128/IAI.01937-06. Epub 2007 Apr 16. Infect Immun. 2007. PMID: 17438028 Free PMC article.
-
Activity-dependent movements of postsynaptic scaffolds at inhibitory synapses.J Neurosci. 2006 Apr 26;26(17):4586-95. doi: 10.1523/JNEUROSCI.5123-05.2006. J Neurosci. 2006. PMID: 16641238 Free PMC article.
-
GIT1 is associated with ADHD in humans and ADHD-like behaviors in mice.Nat Med. 2011 May;17(5):566-72. doi: 10.1038/nm.2330. Epub 2011 Apr 17. Nat Med. 2011. PMID: 21499268
-
p75 regulates Purkinje cell firing by modulating SK channel activity through Rac1.J Biol Chem. 2014 Nov 7;289(45):31458-72. doi: 10.1074/jbc.M114.589937. Epub 2014 Sep 24. J Biol Chem. 2014. PMID: 25253694 Free PMC article.
-
C3 exoenzymes, novel insights into structure and action of Rho-ADP-ribosylating toxins.Naunyn Schmiedebergs Arch Pharmacol. 2007 Feb;374(5-6):347-60. doi: 10.1007/s00210-006-0113-y. Epub 2006 Dec 5. Naunyn Schmiedebergs Arch Pharmacol. 2007. PMID: 17146673 Review.
References
-
- Aktories K, Wegner A. Mechanisms of the cytopathic action of actin-ADP-ribosylating toxins. Mol Microbiol. 1992;6:2905–2908. - PubMed
-
- Aktories K, Barmann M, Ohishi I, Tsuyama S, Jakobs KH, Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986;322:390–392. - PubMed
-
- Aktories K, Braun U, Rosener S, Just I, Hall A. The rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem Biophys Res Commun. 1989;158:209–213. - PubMed
-
- Barry P. JPCalc, a software package for calculating liquid junction potential in patch clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
