The coiled-coil domain of Stat3 is essential for its SH2 domain-mediated receptor binding and subsequent activation induced by epidermal growth factor and interleukin-6
- PMID: 10982829
- PMCID: PMC86266
- DOI: 10.1128/MCB.20.19.7132-7139.2000
The coiled-coil domain of Stat3 is essential for its SH2 domain-mediated receptor binding and subsequent activation induced by epidermal growth factor and interleukin-6
Abstract
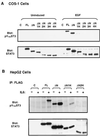
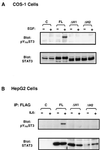
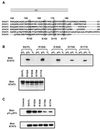
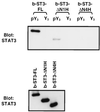
STAT proteins are a family of latent transcription factors that mediate the response to various cytokines and growth factors. Upon stimulation by cytokines, STAT proteins are recruited to the receptors via their SH2 domains, phosphorylated on a specific tyrosine, dimerized, and translocated into the nucleus, where they bind specific DNA sequences and activate the target gene transcription. STATs share highly conserved structures, including an N-domain, a coiled-coil domain, a DNA-binding domain, a linker domain, and an SH2 domain. To investigate the role of the coiled-coil domain, we performed a systematic deletion analysis of the N-domain and each of the alpha-helices and mutagenesis of conserved residues in the coiled-coil region of Stat3. Our results indicate that the coiled-coil domain is essential for Stat3 recruitment to the receptor and the subsequent tyrosine phosphorylation and tyrosine phosphorylation-dependent activities, such as dimer formation, nuclear translocation, and DNA binding, stimulated by epidermal growth factor (EGF) or interleukin-6 (IL-6). Single mutation of Asp170 or, to a lesser extent, Lys177 in alpha-helix 1 diminishes both receptor binding and tyrosine phosphorylation. Furthermore, the Asp170 mutant retains its ability to bind to DNA when phosphorylated on Tyr705 by Src kinase in vitro, implying a functional SH2 domain. Finally, we demonstrate a direct binding of Stat3 to the receptor. Taken together, our data reveal a novel role for the coiled-coil domain that regulates the early events in Stat3 activation and function.
Figures



Similar articles
-
Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells.Mol Cell Biol. 1999 Aug;19(8):5326-38. doi: 10.1128/MCB.19.8.5326. Mol Cell Biol. 1999. PMID: 10409724 Free PMC article.
-
A novel sequence in the coiled-coil domain of Stat3 essential for its nuclear translocation.J Biol Chem. 2003 Aug 1;278(31):29252-60. doi: 10.1074/jbc.M304196200. Epub 2003 May 13. J Biol Chem. 2003. PMID: 12746441
-
Interdomain interaction of Stat3 regulates its Src homology 2 domain-mediated receptor binding activity.J Biol Chem. 2002 May 17;277(20):17556-63. doi: 10.1074/jbc.M105525200. Epub 2002 Feb 28. J Biol Chem. 2002. PMID: 11872739
-
Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway.Biochem J. 1998 Sep 1;334 ( Pt 2)(Pt 2):297-314. doi: 10.1042/bj3340297. Biochem J. 1998. PMID: 9716487 Free PMC article. Review.
-
Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors.Oncogene. 2000 May 15;19(21):2548-56. doi: 10.1038/sj.onc.1203551. Oncogene. 2000. PMID: 10851053 Review.
Cited by
-
STAT3 regulation by S-nitrosylation: implication for inflammatory disease.Antioxid Redox Signal. 2014 Jun 1;20(16):2514-27. doi: 10.1089/ars.2013.5223. Epub 2014 Feb 14. Antioxid Redox Signal. 2014. PMID: 24063605 Free PMC article.
-
Stafia-1: a STAT5a-Selective Inhibitor Developed via Docking-Based Screening of in Silico O-Phosphorylated Fragments.Chemistry. 2020 Jan 2;26(1):148-154. doi: 10.1002/chem.201904147. Epub 2019 Nov 27. Chemistry. 2020. PMID: 31503360 Free PMC article.
-
STAT3 pathway in cancers: Past, present, and future.MedComm (2020). 2022 Mar 23;3(2):e124. doi: 10.1002/mco2.124. eCollection 2022 Jun. MedComm (2020). 2022. PMID: 35356799 Free PMC article. Review.
-
STAT3 Inhibitors: A Novel Insight for Anticancer Therapy of Pancreatic Cancer.Biomolecules. 2022 Oct 9;12(10):1450. doi: 10.3390/biom12101450. Biomolecules. 2022. PMID: 36291659 Free PMC article. Review.
-
Signal transducer and activator of transcription 5B deficiency due to a novel missense mutation in the coiled-coil domain.J Allergy Clin Immunol. 2019 Jan;143(1):413-416.e4. doi: 10.1016/j.jaci.2018.08.032. Epub 2018 Sep 8. J Allergy Clin Immunol. 2019. PMID: 30205186 Free PMC article. No abstract available.
References
-
- Adachi M, Fischer E H, Ihle J, Imai K, Jirik F, Neel B, Pawson T, Shen S, Thomas M, Ullrich A, Zhao Z. Mammalian SH2-containing protein tyrosine phosphatases. Cell. 1996;85:15. - PubMed
-
- Akira S, Nishio Y, Inoue M, Wang X J, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. - PubMed
-
- Becker S, Groner B, Müller C M. Three-dimensional structure of the Stat3β homodimer bound to DNA. Nature. 1998;394:145–151. - PubMed
-
- Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, D'Andrea A, Livingston D M. Cooperation of Stat2 and p300/CBP in signalling induced by interferon-α. Nature. 1996;383:344–347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
