Fast and slow gating relaxations in the muscle chloride channel CLC-1
- PMID: 10962018
- PMCID: PMC2233683
- DOI: 10.1085/jgp.116.3.433
Fast and slow gating relaxations in the muscle chloride channel CLC-1
Abstract
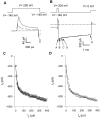
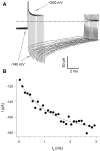
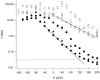
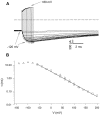
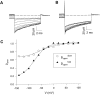
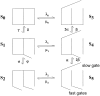
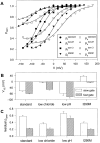
Gating of the muscle chloride channel CLC-1 involves at least two processes evidenced by double-exponential current relaxations when stepping the voltage to negative values. However, there is little information about the gating of CLC-1 at positive voltages. Here, we analyzed macroscopic gating of CLC-1 over a large voltage range (from -160 to +200 mV). Activation was fast at positive voltages but could be easily followed using envelope protocols that employed a tail pulse to -140 mV after stepping the voltage to a certain test potential for increasing durations. Activation was biexponential, demonstrating the presence of two gating processes. Both time constants became exponentially faster at positive voltages. A similar voltage dependence was also seen for the fast gate time constant of CLC-0. The voltage dependence of the time constant of the fast process of CLC-1, tau(f), was steeper than that of the slow one, tau(s) (apparent activation valences were z(f) approximately -0. 79 and z(s) approximately -0.42) such that at +200 mV the two processes became kinetically distinct by almost two orders of magnitude (tau(f) approximately 16 micros, tau(s) approximately 1 ms). This voltage dependence is inconsistent with a previously published gating model for CLC-1 (Fahlke, C., A. Rosenbohm, N. Mitrovic, A.L. George, and R. Rüdel. 1996. Biophys. J. 71:695-706). The kinetic difference at 200 mV allowed us to separate the steady state open probabilities of the two processes assuming that they reflect two parallel (not necessarily independent) gates that have to be open simultaneously to allow ion conduction. Both open probabilities could be described by Boltzmann functions with gating valences around one and with nonzero "offsets" at negative voltages, indicating that the two "gates" never close completely. For comparison with single channel data and to correlate the two gating processes with the two gates of CLC-0, we characterized their voltage, pH(int), and [Cl](ext) dependence, and the dominant myotonia inducing mutation, I290M. Assuming a double-barreled structure of CLC-1, our results are consistent with the identification of the fast and slow gating processes with the single-pore and the common-pore gate, respectively.
Figures


Similar articles
-
Inward rectification in ClC-0 chloride channels caused by mutations in several protein regions.J Gen Physiol. 1997 Aug;110(2):165-71. doi: 10.1085/jgp.110.2.165. J Gen Physiol. 1997. PMID: 9236209 Free PMC article.
-
Drastic reduction of the slow gate of human muscle chloride channel (ClC-1) by mutation C277S.J Physiol. 2001 Aug 1;534(Pt 3):745-52. doi: 10.1111/j.1469-7793.2001.00745.x. J Physiol. 2001. PMID: 11483705 Free PMC article.
-
The muscle chloride channel ClC-1 has a double-barreled appearance that is differentially affected in dominant and recessive myotonia.J Gen Physiol. 1999 Mar;113(3):457-68. doi: 10.1085/jgp.113.3.457. J Gen Physiol. 1999. PMID: 10051520 Free PMC article.
-
Coupling gating with ion permeation in ClC channels.Sci STKE. 2003 Jun 24;2003(188):pe23. doi: 10.1126/stke.2003.188.pe23. Sci STKE. 2003. PMID: 12824475 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
ClC-1 mutations in myotonia congenita patients: insights into molecular gating mechanisms and genotype-phenotype correlation.J Physiol. 2015 Sep 15;593(18):4181-99. doi: 10.1113/JP270358. Epub 2015 Jul 14. J Physiol. 2015. PMID: 26096614 Free PMC article.
-
ClC-1 chloride channels: state-of-the-art research and future challenges.Front Cell Neurosci. 2015 Apr 27;9:156. doi: 10.3389/fncel.2015.00156. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25964741 Free PMC article. Review.
-
ClC-1 Chloride Channel: Inputs on the Structure-Function Relationship of Myotonia Congenita-Causing Mutations.Biomedicines. 2023 Sep 24;11(10):2622. doi: 10.3390/biomedicines11102622. Biomedicines. 2023. PMID: 37892996 Free PMC article. Review.
-
Sequential interaction of chloride and proton ions with the fast gate steer the voltage-dependent gating in ClC-2 chloride channels.J Physiol. 2012 Sep 1;590(17):4239-53. doi: 10.1113/jphysiol.2012.232660. Epub 2012 Jul 2. J Physiol. 2012. PMID: 22753549 Free PMC article.
-
Tryptophan Scanning Mutagenesis Identifies the Molecular Determinants of Distinct Barttin Functions.J Biol Chem. 2015 Jul 24;290(30):18732-43. doi: 10.1074/jbc.M114.625376. Epub 2015 Jun 10. J Biol Chem. 2015. PMID: 26063802 Free PMC article.
References
-
- Fahlke C., Rhodes T.H., Desai R.R., George A.L., Jr. Pore stoichiometry of a voltage-gated chloride channel. Nature. 1998;394:687–690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

