Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120
- PMID: 10954535
- PMCID: PMC116346
- DOI: 10.1128/jvi.74.18.8358-8367.2000
Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120
Abstract
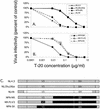
T-20 is a synthetic peptide that potently inhibits replication of human immunodeficiency virus type 1 by interfering with the transition of the transmembrane protein, gp41, to a fusion active state following interactions of the surface glycoprotein, gp120, with CD4 and coreceptor molecules displayed on the target cell surface. Although T-20 is postulated to interact with an N-terminal heptad repeat within gp41 in a trans-dominant manner, we show here that sensitivity to T-20 is strongly influenced by coreceptor specificity. When 14 T-20-naive primary isolates were analyzed for sensitivity to T-20, the mean 50% inhibitory concentration (IC(50)) for isolates that utilize CCR5 for entry (R5 viruses) was 0.8 log(10) higher than the mean IC(50) for CXCR4 (X4) isolates (P = 0. 0055). Using NL4.3-based envelope chimeras that contain combinations of envelope sequences derived from R5 and X4 viruses, we found that determinants of coreceptor specificity contained within the gp120 V3 loop modulate this sensitivity to T-20. The IC(50) for all chimeric envelope viruses containing R5 V3 sequences was 0.6 to 0.8 log(10) higher than that for viruses containing X4 V3 sequences. In addition, we confirmed that the N-terminal heptad repeat of gp41 determines the baseline sensitivity to T-20 and that the IC(50) for viruses containing GIV at amino acid residues 36 to 38 was 1.0 log(10) lower than the IC(50) for viruses containing a G-to-D substitution. The results of this study show that gp120-coreceptor interactions and the gp41 N-terminal heptad repeat independently contribute to sensitivity to T-20. These results have important implications for the therapeutic uses of T-20 as well as for unraveling the complex mechanisms of virus fusion and entry.
Figures



Similar articles
-
Sensitivity of human immunodeficiency virus type 1 to fusion inhibitors targeted to the gp41 first heptad repeat involves distinct regions of gp41 and is consistently modulated by gp120 interactions with the coreceptor.J Virol. 2001 Sep;75(18):8605-14. doi: 10.1128/jvi.75.18.8605-8614.2001. J Virol. 2001. PMID: 11507206 Free PMC article.
-
CD4-induced T-20 binding to human immunodeficiency virus type 1 gp120 blocks interaction with the CXCR4 coreceptor.J Virol. 2004 May;78(10):5448-57. doi: 10.1128/jvi.78.10.5448-5457.2004. J Virol. 2004. PMID: 15113923 Free PMC article.
-
Mutations That Increase the Stability of the Postfusion gp41 Conformation of the HIV-1 Envelope Glycoprotein Are Selected by both an X4 and R5 HIV-1 Virus To Escape Fusion Inhibitors Corresponding to Heptad Repeat 1 of gp41, but the gp120 Adaptive Mutations Differ between the Two Viruses.J Virol. 2019 May 15;93(11):e00142-19. doi: 10.1128/JVI.00142-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894471 Free PMC article.
-
HIV entry inhibitors: mechanisms of action and resistance pathways.J Antimicrob Chemother. 2006 Apr;57(4):619-27. doi: 10.1093/jac/dkl027. Epub 2006 Feb 7. J Antimicrob Chemother. 2006. PMID: 16464888 Review.
-
Progress in targeting HIV-1 entry.Drug Discov Today. 2005 Aug 15;10(16):1085-94. doi: 10.1016/S1359-6446(05)03550-6. Drug Discov Today. 2005. PMID: 16182193 Review.
Cited by
-
Forced virus evolution reveals functional crosstalk between the disulfide bonded region and membrane proximal ectodomain region of HIV-1 gp41.Retrovirology. 2013 Apr 23;10:44. doi: 10.1186/1742-4690-10-44. Retrovirology. 2013. PMID: 23618462 Free PMC article.
-
Analysis of CRISPR/Cas9 Guide RNA Efficiency and Specificity Against Genetically Diverse HIV-1 Isolates.AIDS Res Hum Retroviruses. 2020 Oct;36(10):862-874. doi: 10.1089/AID.2020.0055. Epub 2020 Aug 26. AIDS Res Hum Retroviruses. 2020. PMID: 32640832 Free PMC article.
-
A VRC13-like bNAb response is associated with complex escape pathways in HIV-1 envelope.J Virol. 2024 Mar 19;98(3):e0172023. doi: 10.1128/jvi.01720-23. Epub 2024 Feb 27. J Virol. 2024. PMID: 38412036 Free PMC article.
-
AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges.Nature. 2015 Mar 5;519(7541):87-91. doi: 10.1038/nature14264. Epub 2015 Feb 18. Nature. 2015. PMID: 25707797 Free PMC article.
-
Sequence and structural determinants of human APOBEC3H deaminase and anti-HIV-1 activities.Retrovirology. 2015 Jan 22;12:3. doi: 10.1186/s12977-014-0130-8. Retrovirology. 2015. PMID: 25614027 Free PMC article.
References
-
- Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. - PubMed
-
- Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. - PubMed
-
- Carr C K. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

