A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion
- PMID: 10944212
- PMCID: PMC16876
- DOI: 10.1073/pnas.97.17.9402
A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion
Abstract
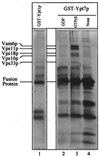
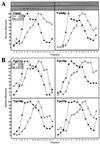
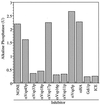
Yeast vacuoles undergo priming, docking, and homotypic fusion, although little has been known of the connections between these reactions. Vacuole-associated Vam2p and Vam6p (Vam2/6p) are components of a 65S complex containing SNARE proteins. Upon priming by Sec18p/NSF and ATP, Vam2/6p is released as a 38S subcomplex that binds Ypt7p to initiate docking. We now report that the 38S complex consists of both Vam2/6p and the class C Vps proteins [Reider, S. E. and Emr, S. D. (1997) Mol. Biol. Cell 8, 2307-2327]. This complex includes Vps33p, a member of the Sec1 family of proteins that bind t-SNAREs. We term this 38S complex HOPS, for homotypic fusion and vacuole protein sorting. This unexpected finding explains how Vam2/6p associates with SNAREs and provides a mechanistic link of the class C Vps proteins to Ypt/Rab action. HOPS initially associates with vacuole SNAREs in "cis" and, after release by priming, hops to Ypt7p, activating this Ypt/Rab switch to initiate docking.
Figures

Similar articles
-
The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein.J Cell Biol. 2000 Mar 20;148(6):1231-8. doi: 10.1083/jcb.148.6.1231. J Cell Biol. 2000. PMID: 10725336 Free PMC article.
-
New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion.J Cell Biol. 2000 Oct 30;151(3):551-62. doi: 10.1083/jcb.151.3.551. J Cell Biol. 2000. PMID: 11062257 Free PMC article.
-
A new role for a SNARE protein as a regulator of the Ypt7/Rab-dependent stage of docking.Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):8889-91. doi: 10.1073/pnas.160269997. Proc Natl Acad Sci U S A. 2000. PMID: 10908678 Free PMC article.
-
Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles.Annu Rev Cell Dev Biol. 2010;26:115-36. doi: 10.1146/annurev-cellbio-100109-104131. Annu Rev Cell Dev Biol. 2010. PMID: 20521906 Review.
-
Involvement of LMA1 and GATE-16 family members in intracellular membrane dynamics.Biochim Biophys Acta. 2003 Aug 18;1641(2-3):145-56. doi: 10.1016/s0167-4889(03)00086-7. Biochim Biophys Acta. 2003. PMID: 12914955 Review.
Cited by
-
Better Together: Current Insights Into Phagosome-Lysosome Fusion.Front Immunol. 2021 Feb 25;12:636078. doi: 10.3389/fimmu.2021.636078. eCollection 2021. Front Immunol. 2021. PMID: 33717183 Free PMC article. Review.
-
Sphingolipids containing very long-chain fatty acids regulate Ypt7 function during the tethering stage of vacuole fusion.J Biol Chem. 2024 Nov;300(11):107808. doi: 10.1016/j.jbc.2024.107808. Epub 2024 Sep 21. J Biol Chem. 2024. PMID: 39307308 Free PMC article.
-
Rab GTPase regulation of retromer-mediated cargo export during endosome maturation.Mol Biol Cell. 2012 Jul;23(13):2505-15. doi: 10.1091/mbc.E11-11-0915. Epub 2012 May 16. Mol Biol Cell. 2012. PMID: 22593205 Free PMC article.
-
A tethering complex dimer catalyzes trans-SNARE complex formation in intracellular membrane fusion.Bioarchitecture. 2012 Feb 1;2(2):59-69. doi: 10.4161/bioa.20359. Bioarchitecture. 2012. PMID: 22754631 Free PMC article.
-
Sec17/Sec18 can support membrane fusion without help from completion of SNARE zippering.Elife. 2021 May 4;10:e67578. doi: 10.7554/eLife.67578. Elife. 2021. PMID: 33944780 Free PMC article.
References
-
- Rothman J H, Stevens T. Cell. 1986;47:1041–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

