Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system
- PMID: 10944190
- PMCID: PMC27824
- DOI: 10.1073/pnas.170128997
Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system
Abstract
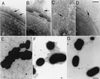
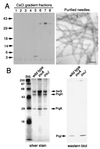
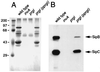
Many bacterial pathogens of plants and animals have evolved a specialized protein-secretion system termed type III to deliver bacterial proteins into host cells. These proteins stimulate or interfere with host cellular functions for the pathogen's benefit. The Salmonella typhimurium pathogenicity island 1 encodes one of these systems that mediates this bacterium's ability to enter nonphagocytic cells. Several components of this type III secretion system are organized in a supramolecular structure termed the needle complex. This structure is made of discrete substructures including a base that spans both membranes and a needle-like projection that extends outward from the bacterial surface. We demonstrate here that the type III secretion export apparatus is required for the assembly of the needle substructure but is dispensable for the assembly of the base. We show that the length of the needle segment is determined by the type III secretion associated protein InvJ. We report that InvG, PrgH, and PrgK constitute the base and that PrgI is the main component of the needle of the type III secretion complex. PrgI homologs are present in type III secretion systems from bacteria pathogenic for animals but are absent from bacteria pathogenic for plants. We hypothesize that the needle component may establish the specificity of type III secretion systems in delivering proteins into either plant or animal cells.
Figures


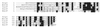

Similar articles
-
Synthesis and localization of the Salmonella SPI-1 type III secretion needle complex proteins PrgI and PrgJ.J Bacteriol. 2003 Jun;185(11):3480-3. doi: 10.1128/JB.185.11.3480-3483.2003. J Bacteriol. 2003. PMID: 12754250 Free PMC article.
-
Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex.J Bacteriol. 2001 Feb;183(4):1159-67. doi: 10.1128/JB.183.4.1159-1167.2001. J Bacteriol. 2001. PMID: 11157927 Free PMC article.
-
Contribution of Salmonella typhimurium type III secretion components to needle complex formation.Proc Natl Acad Sci U S A. 2000 Sep 26;97(20):11008-13. doi: 10.1073/pnas.200209497. Proc Natl Acad Sci U S A. 2000. PMID: 10984518 Free PMC article.
-
Assembly of the type III secretion needle complex of Salmonella typhimurium.Microbes Infect. 2002 Jan;4(1):75-82. doi: 10.1016/s1286-4579(01)01512-x. Microbes Infect. 2002. PMID: 11825778 Review.
-
The invasion-associated type III secretion system of Salmonella typhimurium: common and unique features.Cell Mol Life Sci. 2000 Jul;57(7):1033-49. doi: 10.1007/PL00000743. Cell Mol Life Sci. 2000. PMID: 10961343 Free PMC article. Review.
Cited by
-
Organization and coordinated assembly of the type III secretion export apparatus.Proc Natl Acad Sci U S A. 2010 Oct 12;107(41):17745-50. doi: 10.1073/pnas.1008053107. Epub 2010 Sep 27. Proc Natl Acad Sci U S A. 2010. PMID: 20876096 Free PMC article.
-
Proteolytic cleavage of the FlhB homologue YscU of Yersinia pseudotuberculosis is essential for bacterial survival but not for type III secretion.J Bacteriol. 2002 Aug;184(16):4500-9. doi: 10.1128/JB.184.16.4500-4509.2002. J Bacteriol. 2002. PMID: 12142420 Free PMC article.
-
Assembly and architecture of the type III secretion sorting platform.Proc Natl Acad Sci U S A. 2022 Dec 20;119(51):e2218010119. doi: 10.1073/pnas.2218010119. Epub 2022 Dec 13. Proc Natl Acad Sci U S A. 2022. PMID: 36512499 Free PMC article.
-
Synthesis and localization of the Salmonella SPI-1 type III secretion needle complex proteins PrgI and PrgJ.J Bacteriol. 2003 Jun;185(11):3480-3. doi: 10.1128/JB.185.11.3480-3483.2003. J Bacteriol. 2003. PMID: 12754250 Free PMC article.
-
Assembly of the type III secretion apparatus of enteropathogenic Escherichia coli.J Bacteriol. 2006 Apr;188(8):2801-11. doi: 10.1128/JB.188.8.2801-2811.2006. J Bacteriol. 2006. PMID: 16585741 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

