Gut-enriched Krüppel-like factor represses cyclin D1 promoter activity through Sp1 motif
- PMID: 10908361
- PMCID: PMC102679
- DOI: 10.1093/nar/28.15.2969
Gut-enriched Krüppel-like factor represses cyclin D1 promoter activity through Sp1 motif
Abstract
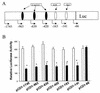
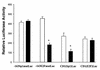
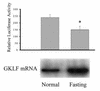
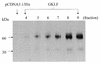
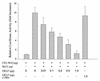
Cancer cells differ from normal cells in many characteristics including loss of differentiation and uninhibited cell proliferation. Recent studies have focused on the identification of factors contributing to cell growth and differentiation. Gut-enriched Krüppel-like factor (GKLF or KLF4) is a newly identified eukaryotic transcription factor and has been shown to play a role in regulating growth arrest. We have previously shown that GKLF mRNA levels were significantly decreased in colon cancer tissues, and that over-expression of GKLF in colonic adenocarcinoma cells (HT-29) resulted in reduction of cyclin D1 (CD1) mRNA and protein levels. The current study was undertaken to determine the mechanisms by which GKLF inhibited CD1 expression. In a transient transfection system, GKLF suppressed CD1 promoter activity by 55%. Sequential deletion and site-directed mutation analysis of the CD1 promoter have identified the sequence between -141 and -66, a region containing an Sp1 response element, to be essential for GKLF function. By electrophoretic mobility gel shift assay, recombinant GKLF and nuclear extracts from HT-29 cells were found to bind to the Sp1 motif on the CD1 promoter. The inhibitory effect of GKLF on the CD1 promoter activity was completely abolished by excessive amount of Sp1 DNA and GKLF significantly reduced the stimulatory function of Sp1 suggesting that GKLF and Sp1 may compete for the same binding site on the CD1 promoter. These results indicate that GKLF is a transcriptional repressor of the CD1 gene and that the inhibitory effect of GKLF is, in part, mediated by interaction with the Sp1 binding domain on its promoter.
Figures

Similar articles
-
Gut-enriched Kruppel-like factor represses ornithine decarboxylase gene expression and functions as checkpoint regulator in colonic cancer cells.J Biol Chem. 2002 Nov 29;277(48):46831-9. doi: 10.1074/jbc.M204816200. Epub 2002 Sep 23. J Biol Chem. 2002. PMID: 12297499
-
Role of gut-enriched Krüppel-like factor in colonic cell growth and differentiation.Am J Physiol Gastrointest Liver Physiol. 2000 Oct;279(4):G806-14. doi: 10.1152/ajpgi.2000.279.4.G806. Am J Physiol Gastrointest Liver Physiol. 2000. PMID: 11005769
-
STAT1 is required for IFN-gamma-mediated gut-enriched Krüppel-like factor expression.Exp Cell Res. 2002 Nov 15;281(1):19-27. doi: 10.1006/excr.2002.5633. Exp Cell Res. 2002. PMID: 12441126
-
The gut-enriched Krüppel-like factor suppresses the activity of the CYP1A1 promoter in an Sp1-dependent fashion.J Biol Chem. 1998 Jul 10;273(28):17917-25. doi: 10.1074/jbc.273.28.17917. J Biol Chem. 1998. PMID: 9651398 Free PMC article.
-
Synergistic activation of the rat laminin gamma1 chain promoter by the gut-enriched Kruppel-like factor (GKLF/KLF4) and Sp1.Nucleic Acids Res. 2002 Jun 1;30(11):2270-9. doi: 10.1093/nar/30.11.2270. Nucleic Acids Res. 2002. PMID: 12034813 Free PMC article.
Cited by
-
A peroxisome proliferator-activated receptor ligand MCC-555 imparts anti-proliferative response in pancreatic cancer cells by PPARgamma-independent up-regulation of KLF4.Toxicol Appl Pharmacol. 2012 Sep 1;263(2):225-32. doi: 10.1016/j.taap.2012.06.014. Epub 2012 Jun 30. Toxicol Appl Pharmacol. 2012. PMID: 22750490 Free PMC article.
-
Convergence of the thyroid hormone and gut-enriched Krüppel-like factor pathways in the context of enterocyte differentiation.J Gastrointest Surg. 2003 Dec;7(8):1053-61; discussion 1061. doi: 10.1016/j.gassur.2003.09.006. J Gastrointest Surg. 2003. PMID: 14675715
-
Characterization of increased mucus production of HT29-MTX-E12 cells grown under Semi-Wet interface with Mechanical Stimulation.PLoS One. 2021 Dec 20;16(12):e0261191. doi: 10.1371/journal.pone.0261191. eCollection 2021. PLoS One. 2021. PMID: 34928974 Free PMC article.
-
The epidermis of grhl3-null mice displays altered lipid processing and cellular hyperproliferation.Organogenesis. 2005 Apr;2(2):33-5. doi: 10.4161/org.2.2.2167. Organogenesis. 2005. PMID: 19521564 Free PMC article.
-
GINS complex subunit 4, a prognostic biomarker and reversely mediated by Krüppel-like factor 4, promotes the growth of colorectal cancer.Cancer Sci. 2020 Apr;111(4):1203-1217. doi: 10.1111/cas.14341. Epub 2020 Mar 17. Cancer Sci. 2020. PMID: 32012389 Free PMC article.
References
-
- Black A.R., Jensen,D., Lin,S.Y. and Azizkhan,J.C. (1999) J. Biol. Chem., 274, 1207–1215. - PubMed
-
- Feng W.C., Southwood,C.M. and Bieker,J.J. (1994) J. Biol. Chem., 269, 1493–1500. - PubMed
-
- Garrett-Sinha L.A., Eberspaecher,H., Seldin,M.F. and Crombrugghe,B.D. (1996) J. Biol. Chem., 271, 31384–31390. - PubMed
-
- Shields J.M., Christy,R.J. and Yang,V.W. (1996) J. Biol. Chem., 71, 20009–20017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

