The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency
- PMID: 10906198
- PMCID: PMC112265
- DOI: 10.1128/jvi.74.16.7451-7461.2000
The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency
Abstract
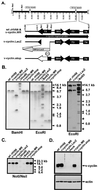
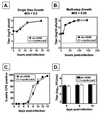
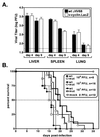
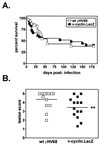
Gamma-2 herpesviruses encode a homolog of mammalian D-type cyclins. The v-cyclin encoded by murine gammaherpesvirus 68 (gammaHV68) induces cell cycle progression and is an oncogene (L. F. van Dyk, J. L. Hess, J. D. Katz, M. Jacoby, S. H. Speck, and H. W. Virgin IV, J. Virol. 73:5110-5122, 1999). However, the role of the pro-proliferative v-cyclins in gamma-2 herpesvirus pathogenesis is not known. Here we report the generation and characterization of a gammaHV68 v-cyclin mutant (v-cyclin.LacZ) that is unable to express a functional v-cyclin protein. Notably, although the gammaHV68 v-cyclin is expressed from an early-late lytic transcript, v-cyclin. LacZ replicated normally in fibroblasts in vitro and during acute infection in the spleen, liver, and lungs in vivo. Moreover, v-cyclin.LacZ exhibited wild-type (wt) virulence in mice with severe combined immunodeficiency. In addition, in a model of gammaHV68-induced chronic disease in mice lacking the gamma interferon receptor (IFNgammaR(-/-)), v-cyclin.LacZ virus was similar to wt gammaHV68 in terms of the incidence of mortality and vasculitis. Further analysis revealed that the frequencies of splenocytes and peritoneal cells harboring the latent gammaHV68 genome in normal and B-cell-deficient mice infected with wt gammaHV68 or v-cyclin.LacZ were very similar. However, v-cyclin.LacZ was significantly compromised in its capacity to reactivate from latency. This phenotype was conclusively mapped to the v-cyclin gene by (i) generating a marker rescue virus (v-cyclin.MR) from the v-cyclin.LacZ mutant, which restored the frequency of cells in which virus reactivated from latency to the levels observed with wt gammaHV68; and (ii) generating a second v-cyclin mutant virus containing a translation stop codon within the v-cyclin gene (v-cyclin.stop), which was compromised in reactivation from latency. These studies demonstrate that despite expression as a lytic cycle gene, the pro-proliferative gammaHV68 v-cyclin is not required for gammaHV68 replication either in vitro or during acute infection in vivo but rather is a critical determinant of reactivation from latency.
Figures



Similar articles
-
Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency.J Virol. 2000 Feb;74(4):1973-84. doi: 10.1128/jvi.74.4.1973-1984.2000. J Virol. 2000. PMID: 10644370 Free PMC article.
-
Identification of an Rta responsive promoter involved in driving gammaHV68 v-cyclin expression during virus replication.Virology. 2007 Sep 1;365(2):250-9. doi: 10.1016/j.virol.2007.03.021. Epub 2007 May 2. Virology. 2007. PMID: 17477952 Free PMC article.
-
Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation.J Virol. 2002 Feb;76(4):1790-801. doi: 10.1128/jvi.76.4.1790-1801.2002. J Virol. 2002. PMID: 11799175 Free PMC article.
-
Gamma interferon blocks gammaherpesvirus reactivation from latency in a cell type-specific manner.J Virol. 2007 Jun;81(11):6134-40. doi: 10.1128/JVI.00108-07. Epub 2007 Mar 14. J Virol. 2007. PMID: 17360749 Free PMC article. Review.
-
Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection.Curr Opin Immunol. 1999 Aug;11(4):371-9. doi: 10.1016/s0952-7915(99)80063-6. Curr Opin Immunol. 1999. PMID: 10448140 Review.
Cited by
-
RNA-guided gene editing of the murine gammaherpesvirus 68 genome reduces infectious virus production.PLoS One. 2021 Jun 4;16(6):e0252313. doi: 10.1371/journal.pone.0252313. eCollection 2021. PLoS One. 2021. PMID: 34086743 Free PMC article.
-
Immune regulation of viral infection and vice versa.Immunol Res. 2005;32(1-3):293-315. doi: 10.1385/IR:32:1-3:293. Immunol Res. 2005. PMID: 16106080 Review.
-
Ex vivo stimulation of B cells latently infected with gammaherpesvirus 68 triggers reactivation from latency.J Virol. 2005 Apr;79(8):5227-31. doi: 10.1128/JVI.79.8.5227-5231.2005. J Virol. 2005. PMID: 15795307 Free PMC article.
-
A surface groove essential for viral Bcl-2 function during chronic infection in vivo.PLoS Pathog. 2005 Sep;1(1):e10. doi: 10.1371/journal.ppat.0010010. Epub 2005 Sep 30. PLoS Pathog. 2005. PMID: 16201011 Free PMC article.
-
Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell-deficient mice.J Virol. 2002 Nov;76(22):11460-8. doi: 10.1128/jvi.76.22.11460-11468.2002. J Virol. 2002. PMID: 12388707 Free PMC article.
References
-
- Arvanitakis L, Yaseen N, Sharma S. Latent membrane protein-1 induces cyclin D2 expression, pRb hyperphosphorylation, and loss of TGF-beta-1-mediated growth inhibition on EBV-positive B cells. J Immunol. 1995;155:1047–1056. - PubMed
-
- Bosma M, Schuler W, Bosma G. The scid mouse mutant. Curr Top Microbiol Immunol. 1988;137:197–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

