Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity
- PMID: 10887161
- PMCID: PMC316735
Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity
Abstract
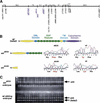
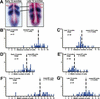
Somitogenesis has been linked both to a molecular clock that controls the oscillation of gene expression in the presomitic mesoderm (PSM) and to Notch pathway signaling. The oscillator, or clock, is thought to create a prepattern of stripes of gene expression that regulates the activity of the Notch pathway that subsequently directs somite border formation. Here, we report that the zebrafish gene after eight (aei) that is required for both somitogenesis and neurogenesis encodes the Notch ligand DeltaD. Additional analysis revealed that stripes of her1 expression oscillate within the PSM and that aei/DeltaD signaling is required for this oscillation. aei/DeltaD expression does not oscillate, indicating that the activity of the Notch pathway upstream of her1 may function within the oscillator itself. Moreover, we found that her1 stripes are expressed in the anlage of consecutive somites, indicating that its expression pattern is not pair-rule. Analysis of her1 expression in aei/DeltaD, fused somites (fss), and aei;fss embryos uncovered a wave-front activity that is capable of continually inducing her1 expression de novo in the anterior PSM in the absence of the oscillation of her1. The wave-front activity, in reference to the clock and wave-front model, is defined as such because it interacts with the oscillator-derived pattern in the anterior PSM and is required for somite morphogenesis. This wave-front activity is blocked in embryos mutant for fss but not aei/DeltaD. Thus, our analysis indicates that the smooth sequence of formation, refinement, and fading of her1 stripes in the PSM is governed by two separate activities.
Figures


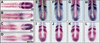


Similar articles
-
The role of Suppressor of Hairless in Notch mediated signalling during zebrafish somitogenesis.Mech Dev. 2003 Sep;120(9):1083-94. doi: 10.1016/s0925-4773(03)00154-0. Mech Dev. 2003. PMID: 14550536
-
her1 and the notch pathway function within the oscillator mechanism that regulates zebrafish somitogenesis.Development. 2002 Mar;129(5):1175-83. doi: 10.1242/dev.129.5.1175. Development. 2002. PMID: 11874913
-
Completing the set of h/E(spl) cyclic genes in zebrafish: her12 and her15 reveal novel modes of expression and contribute to the segmentation clock.Dev Biol. 2007 Apr 15;304(2):615-32. doi: 10.1016/j.ydbio.2007.01.004. Epub 2007 Jan 9. Dev Biol. 2007. PMID: 17274976
-
Oscillatory gene expression and somitogenesis.Wiley Interdiscip Rev Dev Biol. 2012 Sep-Oct;1(5):629-41. doi: 10.1002/wdev.46. Epub 2012 Mar 22. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23799565 Review.
-
Catching a wave: the oscillator and wavefront that create the zebrafish somite.Semin Cell Dev Biol. 2002 Dec;13(6):481-8. doi: 10.1016/s1084952102001015. Semin Cell Dev Biol. 2002. PMID: 12468251 Review.
Cited by
-
Species-specific roles of the Notch ligands, receptors, and targets orchestrating the signaling landscape of the segmentation clock.Front Cell Dev Biol. 2024 Jan 29;11:1327227. doi: 10.3389/fcell.2023.1327227. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38348091 Free PMC article. Review.
-
Generation of segment polarity in the paraxial mesoderm of the zebrafish through a T-box-dependent inductive event.Dev Biol. 2005 Jul 1;283(1):204-14. doi: 10.1016/j.ydbio.2005.04.012. Dev Biol. 2005. PMID: 15921674 Free PMC article.
-
Intrinsic noise, Delta-Notch signalling and delayed reactions promote sustained, coherent, synchronized oscillations in the presomitic mesoderm.J R Soc Interface. 2019 Nov 29;16(160):20190436. doi: 10.1098/rsif.2019.0436. Epub 2019 Nov 27. J R Soc Interface. 2019. PMID: 31771454 Free PMC article.
-
Notch-mediated inhibition of neurogenesis is required for zebrafish spinal cord morphogenesis.Sci Rep. 2019 Jul 10;9(1):9958. doi: 10.1038/s41598-019-46067-1. Sci Rep. 2019. PMID: 31292468 Free PMC article.
-
Noise in the Vertebrate Segmentation Clock Is Boosted by Time Delays but Tamed by Notch Signaling.Cell Rep. 2018 May 15;23(7):2175-2185.e4. doi: 10.1016/j.celrep.2018.04.069. Cell Rep. 2018. PMID: 29768214 Free PMC article.
References
-
- Amacher SL, Kimmel CB. Promoting notochord fate and repressing muscle development in zebrafish. Development. 1998;125:1397–1406. - PubMed
-
- Aoyama H, Asamoto K. Determination of somite cells: Independence of cell differentiation and morphogenesis. Development. 1988;104:15–28. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Aulehla A, Johnson RL. Dynamic expression of lunatic fringe suggests a link between notch signaling and an automomous cellular oscillator driving somite segmentation. Dev Biol. 1999;207:49–61. - PubMed
-
- Bierkamp C, Campos-Ortega JA. A zebrafish homologue of the Drosophila neurogenic gene Notch and its pattern of transcription during early embryogenesis. Mech Dev. 1993;43:87–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
