Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument
- PMID: 10884436
- PMCID: PMC16684
- DOI: 10.1073/pnas.97.14.8146
Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument
Abstract
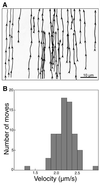
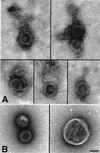
Herpes simplex virus type I (HSV) typically enters peripheral nerve terminals and then travels back along the nerve to reach the neuronal cell body, where it replicates or enters latency. To monitor axoplasmic transport of HSV, we used the giant axon of the squid, Loligo pealei, a well known system for the study of axoplasmic transport. To deliver HSV into the axoplasm, viral particles stripped of their envelopes by detergent were injected into the giant axon, thereby bypassing the infective process. Labeling the viral tegument protein, VP16, with green fluorescent protein allowed viral particles moving inside the axon to be imaged by confocal microscopy. Viral particles moved 2.2 +/- 0.26 micrometer/sec in the retrograde direction, a rate comparable to that of the transport of endogenous organelles and of virus in mammalian neurons in culture. Electron microscopy confirmed that 96% of motile (stripped) viral particles had lost their envelope but retained tegument, and Western blot analysis revealed that these particles had retained protein from capsid but not envelope. We conclude that (i) HSV recruits the squid retrograde transport machinery; (ii) viral tegument and capsid but not envelope are sufficient for this recruitment; and (iii) the giant axon of the squid provides a unique system to dissect the viral components required for transport and to identify the cellular transport mechanisms they recruit.
Figures


Similar articles
-
Characterization of the Herpes Simplex Virus (HSV) Tegument Proteins That Bind to gE/gI and US9, Which Promote Assembly of HSV and Transport into Neuronal Axons.J Virol. 2020 Nov 9;94(23):e01113-20. doi: 10.1128/JVI.01113-20. Print 2020 Nov 9. J Virol. 2020. PMID: 32938770 Free PMC article.
-
Anterograde Viral Tracer Herpes Simplex Virus 1 Strain H129 Transports Primarily as Capsids in Cortical Neuron Axons.J Virol. 2020 Mar 31;94(8):e01957-19. doi: 10.1128/JVI.01957-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969440 Free PMC article.
-
Fast anterograde transport of herpes simplex virus: role for the amyloid precursor protein of alzheimer's disease.Aging Cell. 2003 Dec;2(6):305-18. doi: 10.1046/j.1474-9728.2003.00069.x. Aging Cell. 2003. PMID: 14677633 Free PMC article.
-
Transport and egress of herpes simplex virus in neurons.Rev Med Virol. 2008 Jan-Feb;18(1):35-51. doi: 10.1002/rmv.560. Rev Med Virol. 2008. PMID: 17992661 Review.
-
Anterograde transport of α-herpesviruses in neuronal axons.Virology. 2021 Jul;559:65-73. doi: 10.1016/j.virol.2021.02.011. Epub 2021 Mar 4. Virology. 2021. PMID: 33836340 Review.
Cited by
-
Neuroinflammation in Alzheimer's Disease: A Potential Role of Nose-Picking in Pathogen Entry via the Olfactory System?Biomolecules. 2023 Oct 24;13(11):1568. doi: 10.3390/biom13111568. Biomolecules. 2023. PMID: 38002250 Free PMC article. Review.
-
Human cytomegalovirus tegument proteins ppUL82 (pp71) and ppUL35 interact and cooperatively activate the major immediate-early enhancer.J Virol. 2004 Sep;78(17):9512-23. doi: 10.1128/JVI.78.17.9512-9523.2004. J Virol. 2004. PMID: 15308743 Free PMC article.
-
The neuronal host cell factor-binding protein Zhangfei inhibits herpes simplex virus replication.J Virol. 2005 Dec;79(23):14708-18. doi: 10.1128/JVI.79.23.14708-14718.2005. J Virol. 2005. PMID: 16282471 Free PMC article.
-
Analysis of retrograde transport in motor neurons reveals common endocytic carriers for tetanus toxin and neurotrophin receptor p75NTR.J Cell Biol. 2002 Jan 21;156(2):233-9. doi: 10.1083/jcb.200106142. Epub 2002 Jan 21. J Cell Biol. 2002. PMID: 11807088 Free PMC article.
-
Neuronal Subtype Determines Herpes Simplex Virus 1 Latency-Associated-Transcript Promoter Activity during Latency.J Virol. 2018 Jun 13;92(13):e00430-18. doi: 10.1128/JVI.00430-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29643250 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

