Recombinant green fluorescent protein-expressing human cytomegalovirus as a tool for screening antiviral agents
- PMID: 10817714
- PMCID: PMC89918
- DOI: 10.1128/AAC.44.6.1588-1597.2000
Recombinant green fluorescent protein-expressing human cytomegalovirus as a tool for screening antiviral agents
Abstract
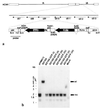
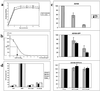
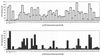
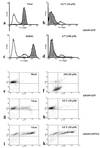
A recombinant human cytomegalovirus (AD169-GFP) expressing green fluorescent protein was generated by homologous recombination. Infection of human fibroblast cultures with AD169-GFP virus produced stable and readily detectable amounts of GFP signals which were quantitated by automated fluorometry. Hereby, high levels of sensitivity and reproducibility could be achieved, compared to those with the conventional plaque reduction assay. Antiviral activities were determined for four reference compounds as well as a set of putative novel cytomegalovirus inhibitors. The results obtained were exactly in line with the known characteristics of reference compounds and furthermore revealed distinct antiviral activities of novel in vitro inhibitors. The fluorometric data could be confirmed by GFP-based flow cytometry and fluorescence microscopy. In addition, laboratory virus variants derived from the recombinant AD169-GFP virus provided further possibilities for study of the characteristics of drug resistance. The GFP-based antiviral assay appeared to be very reliable for measuring virus-inhibitory effects in concentration- and time-dependent fashions and might also be adaptable for high-throughput screenings of cytomegalovirus-specific antiviral agents.
Figures

Similar articles
-
Fluorescence-based antiviral assay for the evaluation of compounds against vaccinia virus, varicella zoster virus and human cytomegalovirus.J Virol Methods. 2008 Jul;151(1):66-73. doi: 10.1016/j.jviromet.2008.03.025. Epub 2008 May 19. J Virol Methods. 2008. PMID: 18490063
-
Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses.J Mol Med (Berl). 2002 Apr;80(4):233-42. doi: 10.1007/s00109-001-0300-8. Epub 2001 Dec 8. J Mol Med (Berl). 2002. PMID: 11976732
-
A fluorescence-based high-throughput screening assay for identifying human cytomegalovirus inhibitors.Methods Mol Biol. 2013;1030:327-34. doi: 10.1007/978-1-62703-484-5_25. Methods Mol Biol. 2013. PMID: 23821279
-
Development of an efficient fluorescence-based microneutralization assay using recombinant human cytomegalovirus strains expressing green fluorescent protein.J Virol Methods. 2004 Sep 15;120(2):207-15. doi: 10.1016/j.jviromet.2004.05.010. J Virol Methods. 2004. PMID: 15288964
-
Antiviral drug susceptibility assays: going with the flow.Antiviral Res. 1999 Aug;43(1):1-21. doi: 10.1016/s0166-3542(99)00039-x. Antiviral Res. 1999. PMID: 10480260 Review.
Cited by
-
In vitro evaluation of the activities of the novel anticytomegalovirus compound AIC246 (letermovir) against herpesviruses and other human pathogenic viruses.Antimicrob Agents Chemother. 2012 Feb;56(2):1135-7. doi: 10.1128/AAC.05908-11. Epub 2011 Nov 21. Antimicrob Agents Chemother. 2012. PMID: 22106211 Free PMC article.
-
The Prolyl Isomerase Pin1 Promotes the Herpesvirus-Induced Phosphorylation-Dependent Disassembly of the Nuclear Lamina Required for Nucleocytoplasmic Egress.PLoS Pathog. 2016 Aug 24;12(8):e1005825. doi: 10.1371/journal.ppat.1005825. eCollection 2016 Aug. PLoS Pathog. 2016. PMID: 27556400 Free PMC article.
-
A DNA-based non-infectious replicon system to study SARS-CoV-2 RNA synthesis.Comput Struct Biotechnol J. 2022;20:5193-5202. doi: 10.1016/j.csbj.2022.08.044. Epub 2022 Aug 30. Comput Struct Biotechnol J. 2022. PMID: 36059866 Free PMC article.
-
Establishment of a Luciferase-Based Reporter System to Study Aspects of Human Cytomegalovirus Infection, Replication Characteristics, and Antiviral Drug Efficacy.Pathogens. 2024 Jul 31;13(8):645. doi: 10.3390/pathogens13080645. Pathogens. 2024. PMID: 39204245 Free PMC article.
-
Human papillomavirus 16 E7 inactivator of retinoblastoma family proteins complements human cytomegalovirus lacking UL97 protein kinase.Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16823-8. doi: 10.1073/pnas.0901521106. Epub 2009 Sep 15. Proc Natl Acad Sci U S A. 2009. PMID: 19805380 Free PMC article.
References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1998.
-
- Azad R F, Brown-Driver V, Buckheit R W, Jr, Anderson K P. Antiviral activity of a phosphorothioate oligonucleotide complementary to human cytomegalovirus RNA when used in combination with antiviral nucleoside analogs. Antiviral Res. 1995;28:101–111. - PubMed
-
- Bevilacqua F, Davis-Poynter N, Worrallo J, Gower D, Collins P, Darby G. Construction of a herpes simplex virus/varicella-zoster virus (HSV/VZV) thymidine kinase recombinant with the pathogenic potential of HSV and a drug sensitivity profile resembling that of VZV. J Gen Virol. 1995;76:1927–1935. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

