An efficient recombination system for chromosome engineering in Escherichia coli
- PMID: 10811905
- PMCID: PMC18544
- DOI: 10.1073/pnas.100127597
An efficient recombination system for chromosome engineering in Escherichia coli
Abstract
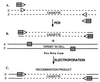
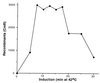
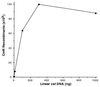
A recombination system has been developed for efficient chromosome engineering in Escherichia coli by using electroporated linear DNA. A defective lambda prophage supplies functions that protect and recombine an electroporated linear DNA substrate in the bacterial cell. The use of recombination eliminates the requirement for standard cloning as all novel joints are engineered by chemical synthesis in vitro and the linear DNA is efficiently recombined into place in vivo. The technology and manipulations required are simple and straightforward. A temperature-dependent repressor tightly controls prophage expression, and, thus, recombination functions can be transiently supplied by shifting cultures to 42 degrees C for 15 min. The efficient prophage recombination system does not require host RecA function and depends primarily on Exo, Beta, and Gam functions expressed from the defective lambda prophage. The defective prophage can be moved to other strains and can be easily removed from any strain. Gene disruptions and modifications of both the bacterial chromosome and bacterial plasmids are possible. This system will be especially useful for the engineering of large bacterial plasmids such as those from bacterial artificial chromosome libraries.
Figures
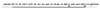
Similar articles
-
A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA.Genomics. 2001 Apr 1;73(1):56-65. doi: 10.1006/geno.2000.6451. Genomics. 2001. PMID: 11352566
-
Recombineering with overlapping single-stranded DNA oligonucleotides: testing a recombination intermediate.Proc Natl Acad Sci U S A. 2003 Jun 10;100(12):7207-12. doi: 10.1073/pnas.1232375100. Epub 2003 May 27. Proc Natl Acad Sci U S A. 2003. PMID: 12771385 Free PMC article.
-
Bacterial artificial chromosome engineering.Methods Mol Biol. 2004;256:89-106. doi: 10.1385/1-59259-753-X:089. Methods Mol Biol. 2004. PMID: 15024162 No abstract available.
-
[Red/ET recombination and its biomedical applications].Sheng Wu Gong Cheng Xue Bao. 2005 May;21(3):502-6. Sheng Wu Gong Cheng Xue Bao. 2005. PMID: 16108384 Review. Chinese.
-
[Recombineering and its application].Yi Chuan Xue Bao. 2003 Oct;30(10):983-8. Yi Chuan Xue Bao. 2003. PMID: 14669518 Review. Chinese.
Cited by
-
Modification of the RpoS network with a synthetic small RNA.Nucleic Acids Res. 2013 Sep;41(17):8332-40. doi: 10.1093/nar/gkt604. Epub 2013 Jul 9. Nucleic Acids Res. 2013. PMID: 23842672 Free PMC article.
-
The Phosin PptA Plays a Negative Role in the Regulation of Antibiotic Production in Streptomyces lividans.Antibiotics (Basel). 2021 Mar 20;10(3):325. doi: 10.3390/antibiotics10030325. Antibiotics (Basel). 2021. PMID: 33804592 Free PMC article.
-
Subcloning plus insertion (SPI)--a novel recombineering method for the rapid construction of gene targeting vectors.J Vis Exp. 2015 Jan 8;(95):e52155. doi: 10.3791/52155. J Vis Exp. 2015. PMID: 25590226 Free PMC article.
-
Comprehensively Characterizing the Thioredoxin Interactome In Vivo Highlights the Central Role Played by This Ubiquitous Oxidoreductase in Redox Control.Mol Cell Proteomics. 2016 Jun;15(6):2125-40. doi: 10.1074/mcp.M115.056440. Epub 2016 Apr 14. Mol Cell Proteomics. 2016. PMID: 27081212 Free PMC article.
-
Fluorescence-based monitoring of ribosome assembly landscapes.BMC Mol Biol. 2015 Feb 25;16:3. doi: 10.1186/s12867-015-0031-y. BMC Mol Biol. 2015. PMID: 25884162 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

