mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors
- PMID: 10811610
- PMCID: PMC384363
- DOI: 10.1093/emboj/19.10.2193
mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors
Abstract
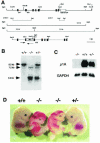
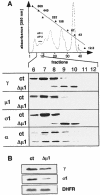
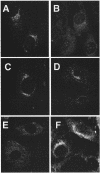
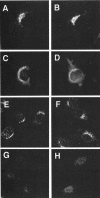
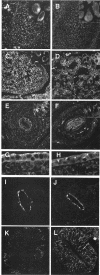
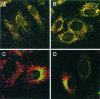
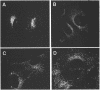
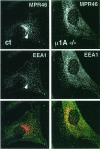
The heterotetrameric AP-1 complex is involved in the formation of clathrin-coated vesicles at the trans-Golgi network (TGN) and interacts with sorting signals in the cytoplasmic tails of cargo molecules. Targeted disruption of the mouse mu1A-adaptin gene causes embryonic lethality at day 13.5. In cells deficient in micro1A-adaptin the remaining AP-1 adaptins do not bind to the TGN. Polarized epithelial cells are the only cells of micro1A-adaptin-deficient embryos that show gamma-adaptin binding to membranes, indicating the formation of an epithelial specific AP-1B complex and demonstrating the absence of additional mu1A homologs. Mannose 6-phosphate receptors are cargo molecules that exit the TGN via AP-1-clathrin-coated vesicles. The steady-state distribution of the mannose 6-phosphate receptors MPR46 and MPR300 in mu1A-deficient cells is shifted to endosomes at the expense of the TGN. MPR46 fails to recycle back from the endosome to the TGN, indicating that AP-1 is required for retrograde endosome to TGN transport of the receptor.
Figures
Similar articles
-
Mu 1A deficiency induces a profound increase in MPR300/IGF-II receptor internalization rate.J Cell Sci. 2001 Dec;114(Pt 24):4469-76. doi: 10.1242/jcs.114.24.4469. J Cell Sci. 2001. PMID: 11792812
-
AP-1 membrane-cytoplasm recycling regulated by mu1A-adaptin.Traffic. 2008 Jan;9(1):121-32. doi: 10.1111/j.1600-0854.2007.00672.x. Epub 2007 Nov 27. Traffic. 2008. PMID: 17988225
-
Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells.J Cell Biol. 2001 Feb 5;152(3):595-606. doi: 10.1083/jcb.152.3.595. J Cell Biol. 2001. PMID: 11157985 Free PMC article.
-
Physiological roles of clathrin adaptor AP complexes: lessons from mutant animals.J Biochem. 2006 Jun;139(6):943-8. doi: 10.1093/jb/mvj120. J Biochem. 2006. PMID: 16788044 Review.
-
Clathrin and adaptors.Biochim Biophys Acta. 1998 Aug 14;1404(1-2):173-93. doi: 10.1016/s0167-4889(98)00056-1. Biochim Biophys Acta. 1998. PMID: 9714795 Review.
Cited by
-
Arabidopsis μ-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth.Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):10318-23. doi: 10.1073/pnas.1300460110. Epub 2013 Jun 3. Proc Natl Acad Sci U S A. 2013. PMID: 23733933 Free PMC article.
-
HAPLESS13, the Arabidopsis μ1 adaptin, is essential for protein sorting at the trans-Golgi network/early endosome.Plant Physiol. 2013 Aug;162(4):1897-910. doi: 10.1104/pp.113.221051. Epub 2013 Jun 13. Plant Physiol. 2013. PMID: 23766365 Free PMC article.
-
Vps26p, a component of retromer, directs the interactions of Vps35p in endosome-to-Golgi retrieval.Mol Biol Cell. 2001 Oct;12(10):3242-56. doi: 10.1091/mbc.12.10.3242. Mol Biol Cell. 2001. PMID: 11598206 Free PMC article.
-
The AP-1 clathrin-adaptor is required for lysosomal enzymes sorting and biogenesis of the contractile vacuole complex in Dictyostelium cells.Mol Biol Cell. 2003 May;14(5):1835-51. doi: 10.1091/mbc.e02-10-0627. Epub 2003 Jan 26. Mol Biol Cell. 2003. PMID: 12802059 Free PMC article.
-
Hsc70 is required for endocytosis and clathrin function in Drosophila.J Cell Biol. 2002 Nov 11;159(3):477-87. doi: 10.1083/jcb.200205086. Epub 2002 Nov 11. J Cell Biol. 2002. PMID: 12427870 Free PMC article.
References
-
- Allan B. and Balch,W. (1999) Protein sorting by directed maturation of Golgi compartments. Science, 285, 63–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

