Role of Rac in controlling the actin cytoskeleton and chemotaxis in motile cells
- PMID: 10805781
- PMCID: PMC25810
- DOI: 10.1073/pnas.97.10.5225
Role of Rac in controlling the actin cytoskeleton and chemotaxis in motile cells
Abstract
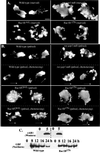
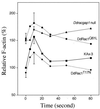
We have used the chemotactic ability of Dictyostelium cells to examine the roles of Rho family members, known regulators of the assembly of F-actin, in cell movement. Wild-type cells polarize with a leading edge enriched in F-actin toward a chemoattractant. Overexpression of constitutively active Dictyostelium Rac1B(61L) or disruption of DdRacGAP1, which encodes a Dictyostelium Rac1 GAP, induces membrane ruffles enriched with actin filaments around the perimeter of the cell and increased levels of F-actin in resting cells. Whereas wild-type cells move linearly toward the cAMP source, Rac1B(61L) and Ddracgap1 null cells make many wrong turns and chemotaxis is inefficient, which presumably results from the unregulated activation of F-actin assembly and pseudopod extension. Cells expressing dominant-negative DdRac1B(17N) do not have a well-defined F-actin-rich leading edge and do not protrude pseudopodia, resulting in very poor cell motility. From these studies and assays examining chemoattractant-mediated F-actin assembly, we suggest DdRac1 regulates the basal levels of F-actin assembly, its dynamic reorganization in response to chemoattractants, and cellular polarity during chemotaxis.
Figures

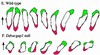
Similar articles
-
Mutant Rac1B expression in Dictyostelium: effects on morphology, growth, endocytosis, development, and the actin cytoskeleton.Cell Motil Cytoskeleton. 2000 Aug;46(4):285-304. doi: 10.1002/1097-0169(200008)46:4<285::AID-CM6>3.0.CO;2-N. Cell Motil Cytoskeleton. 2000. PMID: 10962483
-
WASP-interacting protein is important for actin filament elongation and prompt pseudopod formation in response to a dynamic chemoattractant gradient.Mol Biol Cell. 2006 Oct;17(10):4564-75. doi: 10.1091/mbc.e05-10-0994. Epub 2006 Aug 9. Mol Biol Cell. 2006. PMID: 16899512 Free PMC article.
-
Sphingosine-1-phosphate plays a role in the suppression of lateral pseudopod formation during Dictyostelium discoideum cell migration and chemotaxis.Cell Motil Cytoskeleton. 2004 Dec;59(4):227-41. doi: 10.1002/cm.20035. Cell Motil Cytoskeleton. 2004. PMID: 15476260
-
The molecular genetics of chemotaxis: sensing and responding to chemoattractant gradients.Bioessays. 2000 Jul;22(7):603-15. doi: 10.1002/1521-1878(200007)22:7<603::AID-BIES3>3.0.CO;2-#. Bioessays. 2000. PMID: 10878573 Review.
-
Making all the right moves: chemotaxis in neutrophils and Dictyostelium.Curr Opin Cell Biol. 2004 Feb;16(1):4-13. doi: 10.1016/j.ceb.2003.11.008. Curr Opin Cell Biol. 2004. PMID: 15037299 Review.
Cited by
-
Daydreamer, a Ras effector and GSK-3 substrate, is important for directional sensing and cell motility.Mol Biol Cell. 2013 Jan;24(2):100-14. doi: 10.1091/mbc.E12-04-0271. Epub 2012 Nov 7. Mol Biol Cell. 2013. PMID: 23135995 Free PMC article.
-
Involvement of the cytoskeleton in controlling leading-edge function during chemotaxis.Mol Biol Cell. 2010 Jun 1;21(11):1810-24. doi: 10.1091/mbc.e10-01-0009. Epub 2010 Apr 7. Mol Biol Cell. 2010. PMID: 20375144 Free PMC article.
-
Spatial regulation of the cAMP-dependent protein kinase during chemotactic cell migration.Proc Natl Acad Sci U S A. 2005 Oct 4;102(40):14320-5. doi: 10.1073/pnas.0507072102. Epub 2005 Sep 21. Proc Natl Acad Sci U S A. 2005. PMID: 16176981 Free PMC article.
-
Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression.EMBO J. 2002 Dec 16;21(24):6791-800. doi: 10.1093/emboj/cdf688. EMBO J. 2002. PMID: 12486000 Free PMC article.
-
Inhibition of microRNA suppression of Dishevelled results in Wnt pathway-associated developmental defects in sea urchin.Development. 2018 Nov 30;145(23):dev167130. doi: 10.1242/dev.167130. Development. 2018. PMID: 30389855 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

