Relationship between human immunodeficiency virus type 1 Gag multimerization and membrane binding
- PMID: 10799589
- PMCID: PMC110867
- DOI: 10.1128/jvi.74.11.5142-5150.2000
Relationship between human immunodeficiency virus type 1 Gag multimerization and membrane binding
Abstract
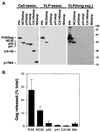
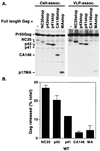
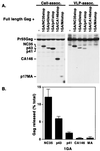
The human immunodeficiency virus type 1 (HIV-1) Gag precursor, Pr55(Gag), is necessary and sufficient for the assembly and release of viruslike particles. Binding of Gag to membrane and Gag multimerization are both essential steps in virus assembly, yet the domains responsible for these events have not been fully defined. In addition, the relationship between membrane binding and Gag-Gag interaction remains to be elucidated. To investigate these issues, we analyzed, in vivo, the membrane-binding and assembly properties of a series of C-terminally truncated Gag mutants. Pr55(Gag) was truncated at the C terminus of matrix (MAstop), between the N- and C-terminal domains of capsid (CA146stop), at the C terminus of capsid (p41stop), at the C terminus of p2 (p43stop), and after the N-terminal 35 amino acids of nucleocapsid (NC35stop). The ability of these truncated Gag molecules to assemble and release viruslike particles and their capacity to copackage into particles when coexpressed with full-length Gag were determined. We demonstrate that the amount of truncated Gag incorporated into particles is incrementally increased by extension from CA146 to NC35, suggesting that multiple sites in this region are involved in Gag multimerization. Using membrane flotation centrifugation, we observe that MA shows significantly reduced membrane binding relative to full-length Gag but that CA146 displays steady-state membrane-binding properties comparable to those of Pr55(Gag). The finding that the CA146 mutant, which contains only matrix and the N-terminal domain of capsid, exhibits levels of steady-state membrane binding equivalent to those of full-length Gag indicates that strong Gag-Gag interaction domains are not required for the efficient binding of HIV-1 Gag to membrane.
Figures



Similar articles
-
Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus.J Virol. 1999 May;73(5):4136-44. doi: 10.1128/JVI.73.5.4136-4144.1999. J Virol. 1999. PMID: 10196310 Free PMC article.
-
Mutation of dileucine-like motifs in the human immunodeficiency virus type 1 capsid disrupts virus assembly, gag-gag interactions, gag-membrane binding, and virion maturation.J Virol. 2006 Aug;80(16):7939-51. doi: 10.1128/JVI.00355-06. J Virol. 2006. PMID: 16873251 Free PMC article.
-
Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain.J Virol. 2000 Jun;74(12):5395-402. doi: 10.1128/jvi.74.12.5395-5402.2000. J Virol. 2000. PMID: 10823843 Free PMC article.
-
Relationship between HIV-1 Gag Multimerization and Membrane Binding.Viruses. 2022 Mar 16;14(3):622. doi: 10.3390/v14030622. Viruses. 2022. PMID: 35337029 Free PMC article. Review.
-
The Assembly of HTLV-1-How Does It Differ from HIV-1?Viruses. 2024 Sep 27;16(10):1528. doi: 10.3390/v16101528. Viruses. 2024. PMID: 39459862 Free PMC article. Review.
Cited by
-
Efavirenz enhances HIV-1 gag processing at the plasma membrane through Gag-Pol dimerization.J Virol. 2013 Mar;87(6):3348-60. doi: 10.1128/JVI.02306-12. Epub 2013 Jan 9. J Virol. 2013. PMID: 23302874 Free PMC article.
-
Plasma membrane rafts play a critical role in HIV-1 assembly and release.Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13925-30. doi: 10.1073/pnas.241320298. Proc Natl Acad Sci U S A. 2001. PMID: 11717449 Free PMC article.
-
Interrelationship between cytoplasmic retroviral Gag concentration and Gag-membrane association.J Mol Biol. 2014 Apr 3;426(7):1611-24. doi: 10.1016/j.jmb.2013.11.025. Epub 2013 Dec 4. J Mol Biol. 2014. PMID: 24316368 Free PMC article.
-
Quantifying protein-protein interactions of peripheral membrane proteins by fluorescence brightness analysis.Biophys J. 2014 Jul 1;107(1):66-75. doi: 10.1016/j.bpj.2014.04.055. Biophys J. 2014. PMID: 24988342 Free PMC article.
-
Kinetics of aggregation with a finite number of particles and application to viral capsid assembly.J Math Biol. 2015 Jun;70(7):1685-705. doi: 10.1007/s00285-014-0819-2. Epub 2014 Aug 8. J Math Biol. 2015. PMID: 25103220
References
-
- Ames J B, Ishima R, Tanaka T, Gordon J I, Stryer L, Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

