Sorting to synaptic-like microvesicles from early and late endosomes requires overlapping but not identical targeting signals
- PMID: 10793153
- PMCID: PMC14885
- DOI: 10.1091/mbc.11.5.1801
Sorting to synaptic-like microvesicles from early and late endosomes requires overlapping but not identical targeting signals
Abstract
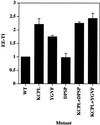
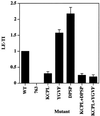
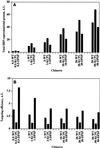
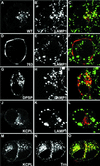
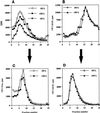
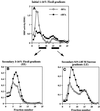
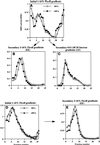
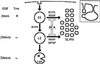
In PC12 neuroendocrine cells, synaptic-like microvesicles (SLMV) are thought to be formed by two pathways. One pathway sorts the proteins to SLMV directly from the plasma membrane (or a specialized domain thereof) in an adaptor protein complex 2-dependent, brefeldin A (BFA)-insensitive manner. Another pathway operates via an endosomal intermediate, involves adaptor protein complex 3, and is BFA sensitive. We have previously shown that when expressed in PC12 cells, HRP-P-selectin chimeras are directed to SLMV mostly via the endosomal, BFA-sensitive route. We have now found that two endosomal intermediates are involved in targeting of HRP-P-selectin chimeras to SLMV. The first intermediate is the early, transferrin-positive, epidermal growth factor-positive endosome, from which exit to SLMV is controlled by the targeting determinants YGVF and KCPL, located within the cytoplasmic domain of P-selectin. The second intermediate is the late, transferrin-negative, epidermal growth factor-positive late endosome, from where HRP-P-selectin chimeras are sorted to SLMV in a YGVF- and DPSP-dependent manner. Both sorting steps, early endosomes to SLMV and late endosomes to SLMV, are affected by BFA. In addition, analysis of double mutants with alanine substitutions of KCPL and YGVF or KCPL and DPSP indicated that chimeras pass sequentially through these intermediates en route both to lysosomes and to SLMV. We conclude that a third site of formation for SLMV, the late endosomes, exists in PC12 cells.
Figures

Similar articles
-
A complex web of signal-dependent trafficking underlies the triorganellar distribution of P-selectin in neuroendocrine PC12 cells.J Cell Biol. 1999 Jun 28;145(7):1419-33. doi: 10.1083/jcb.145.7.1419. J Cell Biol. 1999. PMID: 10385522 Free PMC article.
-
Di-leucine signals mediate targeting of tyrosinase and synaptotagmin to synaptic-like microvesicles within PC12 cells.Mol Biol Cell. 1999 Nov;10(11):3979-90. doi: 10.1091/mbc.10.11.3979. Mol Biol Cell. 1999. PMID: 10564285 Free PMC article.
-
A balance of opposing signals within the cytoplasmic tail controls the lysosomal targeting of P-selectin.J Biol Chem. 1998 Oct 23;273(43):27896-903. doi: 10.1074/jbc.273.43.27896. J Biol Chem. 1998. PMID: 9774401
-
Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic tail.J Biol Chem. 1998 Jan 30;273(5):2729-37. doi: 10.1074/jbc.273.5.2729. J Biol Chem. 1998. PMID: 9446579
-
Secretagogue-triggered transfer of membrane proteins from neuroendocrine secretory granules to synaptic-like microvesicles.Mol Biol Cell. 1999 Aug;10(8):2619-30. doi: 10.1091/mbc.10.8.2619. Mol Biol Cell. 1999. PMID: 10436017 Free PMC article.
Cited by
-
Ligand-induced internalization of the p75 neurotrophin receptor: a slow route to the signaling endosome.J Neurosci. 2003 Apr 15;23(8):3209-20. doi: 10.1523/JNEUROSCI.23-08-03209.2003. J Neurosci. 2003. PMID: 12716928 Free PMC article.
-
Trafficking of vesicular neurotransmitter transporters.Traffic. 2008 Sep;9(9):1425-36. doi: 10.1111/j.1600-0854.2008.00771.x. Epub 2008 May 26. Traffic. 2008. PMID: 18507811 Free PMC article. Review.
-
A conserved mechanism of synaptogyrin localization.Mol Biol Cell. 2001 Aug;12(8):2275-89. doi: 10.1091/mbc.12.8.2275. Mol Biol Cell. 2001. PMID: 11514616 Free PMC article.
-
Rab4 regulates formation of synaptic-like microvesicles from early endosomes in PC12 cells.Mol Biol Cell. 2001 Nov;12(11):3703-15. doi: 10.1091/mbc.12.11.3703. Mol Biol Cell. 2001. PMID: 11694600 Free PMC article.
-
AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes.Mol Biol Cell. 2001 Jul;12(7):2075-85. doi: 10.1091/mbc.12.7.2075. Mol Biol Cell. 2001. PMID: 11452004 Free PMC article.
References
-
- Bauerfeind R, Huttner WB. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol. 1993;5:628–635. - PubMed
-
- Blagoveshchenskaya AD, Hewitt EW, Cutler DF. A balance of opposing signals within the cytoplasmic tail controls the lysosomal targeting of P-selectin. J Biol Chem. 1998a;273:27896–27903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

