Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation
- PMID: 10790433
- PMCID: PMC2213442
- DOI: 10.1084/jem.191.9.1591
Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation
Abstract
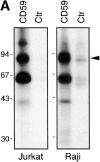
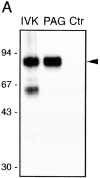
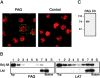
According to a recently proposed hypothesis, initiation of signal transduction via immunoreceptors depends on interactions of the engaged immunoreceptor with glycosphingolipid-enriched membrane microdomains (GEMs). In this study, we describe a novel GEM-associated transmembrane adaptor protein, termed phosphoprotein associated with GEMs (PAG). PAG comprises a short extracellular domain of 16 amino acids and a 397-amino acid cytoplasmic tail containing ten tyrosine residues that are likely phosphorylated by Src family kinases. In lymphoid cell lines and in resting peripheral blood alpha/beta T cells, PAG is expressed as a constitutively tyrosine-phosphorylated protein and binds the major negative regulator of Src kinases, the tyrosine kinase Csk. After activation of peripheral blood alpha/beta T cells, PAG becomes rapidly dephosphorylated and dissociates from Csk. Expression of PAG in COS cells results in recruitment of endogenous Csk, altered Src kinase activity, and impaired phosphorylation of Src-specific substrates. Moreover, overexpression of PAG in Jurkat cells downregulates T cell receptor-mediated activation of the transcription factor nuclear factor of activated T cells. These findings collectively suggest that in the absence of external stimuli, the PAG-Csk complex transmits negative regulatory signals and thus may help to keep resting T cells in a quiescent state.
Figures











Similar articles
-
Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor.Mol Cell Biol. 2003 Mar;23(6):2017-28. doi: 10.1128/MCB.23.6.2017-2028.2003. Mol Cell Biol. 2003. PMID: 12612075 Free PMC article.
-
Positive and Negative Regulatory Roles of C-Terminal Src Kinase (CSK) in FcεRI-Mediated Mast Cell Activation, Independent of the Transmembrane Adaptor PAG/CSK-Binding Protein.Front Immunol. 2018 Aug 2;9:1771. doi: 10.3389/fimmu.2018.01771. eCollection 2018. Front Immunol. 2018. PMID: 30116247 Free PMC article.
-
Revisiting the Timing of Action of the PAG Adaptor Using Quantitative Proteomics Analysis of Primary T Cells.J Immunol. 2015 Dec 1;195(11):5472-81. doi: 10.4049/jimmunol.1501300. Epub 2015 Oct 28. J Immunol. 2015. PMID: 26512138
-
Transmembrane adaptor proteins in membrane microdomains: important regulators of immunoreceptor signaling.Immunol Lett. 2004 Mar 29;92(1-2):43-9. doi: 10.1016/j.imlet.2003.10.013. Immunol Lett. 2004. PMID: 15081526 Review.
-
Spatiotemporal control of cyclic AMP immunomodulation through the PKA-Csk inhibitory pathway is achieved by anchoring to an Ezrin-EBP50-PAG scaffold in effector T cells.FEBS Lett. 2010 Jun 18;584(12):2681-8. doi: 10.1016/j.febslet.2010.04.056. Epub 2010 Apr 24. FEBS Lett. 2010. PMID: 20420835 Review.
Cited by
-
Inhibiting ACK1-mediated phosphorylation of C-terminal Src kinase counteracts prostate cancer immune checkpoint blockade resistance.Nat Commun. 2022 Nov 14;13(1):6929. doi: 10.1038/s41467-022-34724-5. Nat Commun. 2022. PMID: 36376335 Free PMC article.
-
TCR activation kinetics and feedback regulation in primary human T cells.Cell Commun Signal. 2013 Jan 14;11:4. doi: 10.1186/1478-811X-11-4. Cell Commun Signal. 2013. PMID: 23317458 Free PMC article.
-
Systems-wide analysis of BCR signalosomes and downstream phosphorylation and ubiquitylation.Mol Syst Biol. 2015 Jun 2;11(6):810. doi: 10.15252/msb.20145880. Mol Syst Biol. 2015. PMID: 26038114 Free PMC article.
-
Lyn, a Src family kinase, regulates activation of epidermal growth factor receptors in lung adenocarcinoma cells.Mol Cancer. 2013 Jul 16;12:76. doi: 10.1186/1476-4598-12-76. Mol Cancer. 2013. PMID: 23866081 Free PMC article.
-
Lck, Membrane Microdomains, and TCR Triggering Machinery: Defining the New Rules of Engagement.Front Immunol. 2012 Jun 12;3:155. doi: 10.3389/fimmu.2012.00155. eCollection 2012. Front Immunol. 2012. PMID: 22701458 Free PMC article.
References
-
- Tamir I., Cambier J.C. Antigen receptor signalingintegration of protein tyrosine kinase functions. Oncogene. 1998;17:1353–1364. - PubMed
-
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. - PubMed
-
- Xavier R., Seed B. Membrane compartmentation and the response to antigen. Curr. Opin. Immunol. 1999;11:265–269. - PubMed
-
- Brown D.A., London E. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 1998;164:103–114. - PubMed
-
- Harder T., Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell Biol. 1997;9:534–542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

