Conditional expression of a Gi-coupled receptor causes ventricular conduction delay and a lethal cardiomyopathy
- PMID: 10781088
- PMCID: PMC18317
- DOI: 10.1073/pnas.97.9.4826
Conditional expression of a Gi-coupled receptor causes ventricular conduction delay and a lethal cardiomyopathy
Abstract
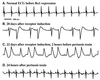
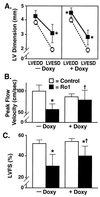
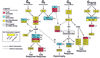
Cardiomyopathy is a major cause of morbidity and mortality. Ventricular conduction delay, as shown by prolonged deflections in the electrocardiogram caused by delayed ventricular contraction (wide QRS complex), is a common feature of cardiomyopathy and is associated with a poor prognosis. Although the G(i)-signaling pathway is up-regulated in certain cardiomyopathies, previous studies suggested this up-regulation was compensatory rather than a potential cause of the disease. Using the tetracycline transactivator system and a modified G(i)-coupled receptor (Ro1), we provide evidence that increased G(i) signaling in mice can result in a lethal cardiomyopathy associated with a wide QRS complex arrhythmia. Induced expression of Ro1 in adult mice resulted in a >90% mortality rate at 16 wk, whereas suppression of Ro1 expression after 8 wk protected mice from further mortality and allowed partial improvement in systolic function. Results of DNA-array analysis of over 6,000 genes from hearts expressing Ro1 are consistent with hyperactive G(i) signaling. DNA-array analysis also identified known markers of cardiomyopathy and hundreds of previously unknown potential diagnostic markers and therapeutic targets for this syndrome. Our system allows cardiomyopathy to be induced and reversed in adult mice, providing an unprecedented opportunity to dissect the role of G(i) signaling in causing cardiac pathology.
Figures



Similar articles
-
Abnormal contraction caused by expression of G(i)-coupled receptor in transgenic model of dilated cardiomyopathy.Am J Physiol Heart Circ Physiol. 2001 Apr;280(4):H1653-9. doi: 10.1152/ajpheart.2001.280.4.H1653. Am J Physiol Heart Circ Physiol. 2001. PMID: 11247776
-
Expression of a Gi-coupled receptor in the heart causes impaired Ca2+ handling, myofilament injury, and dilated cardiomyopathy.Am J Physiol Heart Circ Physiol. 2008 Jan;294(1):H205-12. doi: 10.1152/ajpheart.00829.2007. Epub 2007 Oct 26. Am J Physiol Heart Circ Physiol. 2008. PMID: 17965283 Free PMC article.
-
Conditional expression and signaling of a specifically designed Gi-coupled receptor in transgenic mice.Nat Biotechnol. 1999 Feb;17(2):165-9. doi: 10.1038/6165. Nat Biotechnol. 1999. PMID: 10052353
-
Sarcomeric proteins and inherited cardiomyopathies.Cardiovasc Res. 2008 Mar 1;77(4):659-66. doi: 10.1093/cvr/cvm084. Epub 2007 Dec 4. Cardiovasc Res. 2008. PMID: 18056765 Review.
-
[Arrhythmia-induced dilated cardiomyopathies].Bull Acad Natl Med. 2006 Jun;190(6):1225-35; discussion 1235-6. Bull Acad Natl Med. 2006. PMID: 17195405 Review. French.
Cited by
-
Morphological and behavioral changes in the pathogenesis of a novel mouse model of communicating hydrocephalus.PLoS One. 2012;7(1):e30159. doi: 10.1371/journal.pone.0030159. Epub 2012 Jan 24. PLoS One. 2012. PMID: 22291910 Free PMC article.
-
Search for the "ideal analgesic" in pain treatment by engineering the mu-opioid receptor.IUBMB Life. 2010 Feb;62(2):103-11. doi: 10.1002/iub.292. IUBMB Life. 2010. PMID: 20039371 Free PMC article. Review.
-
Prominent roles for odorant receptor coding sequences in allelic exclusion.Cell. 2007 Nov 30;131(5):1009-17. doi: 10.1016/j.cell.2007.10.050. Cell. 2007. PMID: 18045541 Free PMC article.
-
The GPR17 Receptor-A Promising Goal for Therapy and a Potential Marker of the Neurodegenerative Process in Multiple Sclerosis.Int J Mol Sci. 2020 Mar 8;21(5):1852. doi: 10.3390/ijms21051852. Int J Mol Sci. 2020. PMID: 32182666 Free PMC article. Review.
-
Gap junction remodeling and spironolactone-dependent reverse remodeling in the hypertrophied heart.Circ Res. 2009 Feb 13;104(3):365-71. doi: 10.1161/CIRCRESAHA.108.184044. Epub 2008 Dec 18. Circ Res. 2009. PMID: 19096029 Free PMC article.
References
-
- Schwartz K, Mercadier J-J. Curr Opin Cardiol. 1996;11:227–236. - PubMed
-
- Cohn J N, Bristow M R, Chien K R, Colucci W S, Frazier O H, Leinwand L A, Lorell B H, Moss A J, Sonnenblick E H, Walsh R A, et al. Circulation. 1997;95:766–770. - PubMed
-
- Manolio T A, Baughman K L, Rodeheffer R, Pearson T A, Bristow J D, Michels V V, Abelmann W H, Harlan W R. Am J Cardiol. 1992;69:1458–1466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

