The role of members of the pertussis toxin-sensitive family of G proteins in coupling receptors to the activation of the G protein-gated inwardly rectifying potassium channel
- PMID: 10779550
- PMCID: PMC25883
- DOI: 10.1073/pnas.080572297
The role of members of the pertussis toxin-sensitive family of G proteins in coupling receptors to the activation of the G protein-gated inwardly rectifying potassium channel
Abstract
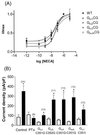
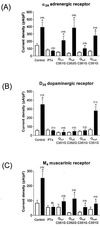
Inwardly rectifying potassium (K(+)) channels gated by G proteins (Kir3.x family) are widely distributed in neuronal, atrial, and endocrine tissues and play key roles in generating late inhibitory postsynaptic potentials, slowing the heart rate and modulating hormone release. They are directly activated by G(betagamma) subunits released from G protein heterotrimers of the G(i/o) family upon appropriate receptor stimulation. Here we examine the role of isoforms of pertussis toxin (PTx)-sensitive G protein alpha subunits (G(ialpha1-3) and G(oalphaA)) in mediating coupling between various receptor systems (A(1), alpha(2A), D(2S), M(4), GABA(B)1a+2, and GABA(B)1b+2) and the cloned counterpart of the neuronal channel (Kir3.1+3.2A). The expression of mutant PTx-resistant G(i/oalpha) subunits in PTx-treated HEK293 cells stably expressing Kir3.1+3.2A allows us to selectively investigate that coupling. We find that, for those receptors (A(1), alpha(2A)) known to interact with all isoforms, G(ialpha1-3) and G(oalphaA) can all support a significant degree of coupling to Kir3.1+3.2A. The M(4) receptor appears to preferentially couple to G(ialpha2) while another group of receptors (D(2S), GABA(B)1a+2, GABA(B)1b+2) activates the channel predominantly through G(betagamma) liberated from G(oA) heterotrimers. Interestingly, we have also found a distinct difference in G protein coupling between the two splice variants of GABA(B)1. Our data reveal selective pathways of receptor activation through different G(i/oalpha) isoforms for stimulation of the G protein-gated inwardly rectifying K(+) channel.
Figures


Similar articles
-
Agonist unbinding from receptor dictates the nature of deactivation kinetics of G protein-gated K+ channels.Proc Natl Acad Sci U S A. 2003 May 13;100(10):6239-44. doi: 10.1073/pnas.1037595100. Epub 2003 Apr 28. Proc Natl Acad Sci U S A. 2003. PMID: 12719528 Free PMC article.
-
The G protein alpha subunit has a key role in determining the specificity of coupling to, but not the activation of, G protein-gated inwardly rectifying K(+) channels.J Biol Chem. 2000 Jan 14;275(2):921-9. doi: 10.1074/jbc.275.2.921. J Biol Chem. 2000. PMID: 10625628
-
Pertussis-toxin-sensitive Galpha subunits selectively bind to C-terminal domain of neuronal GIRK channels: evidence for a heterotrimeric G-protein-channel complex.Mol Cell Neurosci. 2005 Feb;28(2):375-89. doi: 10.1016/j.mcn.2004.10.009. Mol Cell Neurosci. 2005. PMID: 15691717
-
Measuring the modulatory effects of RGS proteins on GIRK channels.Methods Enzymol. 2004;389:131-54. doi: 10.1016/S0076-6879(04)89009-8. Methods Enzymol. 2004. PMID: 15313564 Review.
-
GABAB receptor coupling to G-proteins and ion channels.Adv Pharmacol. 2010;58:123-47. doi: 10.1016/S1054-3589(10)58006-2. Adv Pharmacol. 2010. PMID: 20655481 Review.
Cited by
-
The Role of Inhibitory G Proteins and Regulators of G Protein Signaling in the in vivo Control of Heart Rate and Predisposition to Cardiac Arrhythmias.Front Physiol. 2012 Apr 24;3:96. doi: 10.3389/fphys.2012.00096. eCollection 2012. Front Physiol. 2012. PMID: 22783193 Free PMC article.
-
Generation of Gαi knock-out HEK293 cells illuminates Gαi-coupling diversity of GPCRs.Commun Biol. 2023 Jan 28;6(1):112. doi: 10.1038/s42003-023-04465-2. Commun Biol. 2023. PMID: 36709222 Free PMC article.
-
Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor.Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1426-31. doi: 10.1073/pnas.0336365100. Epub 2003 Jan 27. Proc Natl Acad Sci U S A. 2003. PMID: 12552127 Free PMC article.
-
Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells.Proc Natl Acad Sci U S A. 2005 Dec 20;102(51):18706-11. doi: 10.1073/pnas.0504778102. Epub 2005 Dec 13. Proc Natl Acad Sci U S A. 2005. PMID: 16352729 Free PMC article.
-
Modulatory role of adenosine receptors in insect motor nerve terminals.Neurochem Res. 2003 Apr;28(3-4):617-24. doi: 10.1023/a:1022893928104. Neurochem Res. 2003. PMID: 12675152
References
-
- Kubo Y, Reuveny E, Slesinger P A, Jan Y N, Jan L Y. Nature (London) 1993;364:802–806. - PubMed
-
- Lesage F, Duprat F, Fink M, Guillemare E, Coppola T, Lazdunski M, Hugnot J-P. FEBS Lett. 1994;353:37–42. - PubMed
-
- Krapivinsky G, Gordon E A, Wickman K, Velimirovic B, Krapivinsky L, Clapham D E. Nature (London) 1995;374:135–141. - PubMed
-
- Hedin K E, Lim N F, Clapham D E. Neuron. 1996;16:423–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

