Identification of the Saccharomyces cerevisiae RNA:pseudouridine synthase responsible for formation of psi(2819) in 21S mitochondrial ribosomal RNA
- PMID: 10756195
- PMCID: PMC103309
- DOI: 10.1093/nar/28.9.1941
Identification of the Saccharomyces cerevisiae RNA:pseudouridine synthase responsible for formation of psi(2819) in 21S mitochondrial ribosomal RNA
Abstract
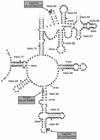
So far, four RNA:pseudouridine (Psi)-synthases have been identified in yeast Saccharomyces cerevisiae. Together, they act on cytoplasmic and mitochondrial tRNAs, U2 snRNA and rRNAs from cytoplasmic ribosomes. However, RNA:Psi-synthases responsible for several U-->Psi conversions in tRNAs and UsnRNAs remained to be identified. Based on conserved amino-acid motifs in already characterised RNA:Psi-synthases, four additional open reading frames (ORFs) encoding putative RNA:Psi-synthases were identified in S.cerevisiae. Upon disruption of one of them, the YLR165c ORF, we found that the unique Psi residue normally present in the fully matured mitochondrial rRNAs (Psi(2819)in 21S rRNA) was missing, while Psi residues at all the tested pseudo-uridylation sites in cytoplasmic and mitochondrial tRNAs and in nuclear UsnRNAs were retained. The selective U-->Psi conversion at position 2819 in mitochondrial 21S rRNA was restored when the deleted yeast strain was transformed by a plasmid expressing the wild-type YLR165c ORF. Complementation was lost after point mutation (D71-->A) in the postulated active site of the YLR165c-encoded protein, indicating the direct role of the YLR165c protein in Psi(2819)synthesis in mitochondrial 21S rRNA. Hence, for nomenclature homogeneity the YLR165c ORF was renamed PUS5 and the corresponding RNA:Psi-synthase Pus5p. As already noticed for other mitochondrial RNA modification enzymes, no canonical mitochondrial targeting signal was identified in Pus5p. Our results also show that Psi(2819)in mitochondrial 21S rRNA is not essential for cell viability.
Figures



Similar articles
-
The yeast gene YNL292w encodes a pseudouridine synthase (Pus4) catalyzing the formation of psi55 in both mitochondrial and cytoplasmic tRNAs.Nucleic Acids Res. 1997 Nov 15;25(22):4493-9. doi: 10.1093/nar/25.22.4493. Nucleic Acids Res. 1997. PMID: 9358157 Free PMC article.
-
Pseudouridine mapping in the Saccharomyces cerevisiae spliceosomal U small nuclear RNAs (snRNAs) reveals that pseudouridine synthase pus1p exhibits a dual substrate specificity for U2 snRNA and tRNA.Mol Cell Biol. 1999 Mar;19(3):2142-54. doi: 10.1128/MCB.19.3.2142. Mol Cell Biol. 1999. PMID: 10022901 Free PMC article.
-
Identification and characterization of the tRNA:Psi 31-synthase (Pus6p) of Saccharomyces cerevisiae.J Biol Chem. 2001 Sep 14;276(37):34934-40. doi: 10.1074/jbc.M103131200. Epub 2001 Jun 13. J Biol Chem. 2001. PMID: 11406626
-
Pseudouridine: still mysterious, but never a fake (uridine)!RNA Biol. 2014;11(12):1540-54. doi: 10.4161/15476286.2014.992278. RNA Biol. 2014. PMID: 25616362 Free PMC article. Review.
-
Pseudouridine in RNA: what, where, how, and why.IUBMB Life. 2000 May;49(5):341-51. doi: 10.1080/152165400410182. IUBMB Life. 2000. PMID: 10902565 Review.
Cited by
-
A second function for pseudouridine synthases: A point mutant of RluD unable to form pseudouridines 1911, 1915, and 1917 in Escherichia coli 23S ribosomal RNA restores normal growth to an RluD-minus strain.RNA. 2001 Jul;7(7):990-8. doi: 10.1017/s1355838201000243. RNA. 2001. PMID: 11453071 Free PMC article.
-
A compendium of human mitochondrial gene expression machinery with links to disease.Environ Mol Mutagen. 2010 Jun;51(5):360-79. doi: 10.1002/em.20571. Environ Mol Mutagen. 2010. PMID: 20544879 Free PMC article. Review.
-
Mouse pseudouridine synthase 1: gene structure and alternative splicing of pre-mRNA.Biochem J. 2000 Dec 1;352 Pt 2(Pt 2):465-73. Biochem J. 2000. PMID: 11085940 Free PMC article.
-
Dietary serine-microbiota interaction enhances chemotherapeutic toxicity without altering drug conversion.Nat Commun. 2020 May 22;11(1):2587. doi: 10.1038/s41467-020-16220-w. Nat Commun. 2020. PMID: 32444616 Free PMC article.
-
Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells.Nature. 2014 Nov 6;515(7525):143-6. doi: 10.1038/nature13802. Epub 2014 Sep 5. Nature. 2014. PMID: 25192136 Free PMC article.
References
-
- Grosjean H., Sprinzl,M. and Steinberg,S. (1995) Biochimie, 77, 139–141. - PubMed
-
- Massenet S., Mougin,A. and Branlant,C. (1998) In Grosjean,H. and Benne,R. (eds), The Modification and Editing of RNA. ASM Press, Washington DC, pp. 201–228.
-
- Ofengand J. and Fournier,M.J. (1998) In Grosjean,H. and Benne,R. (eds), The Modification and Editing of RNA. ASM Press, Washington DC, pp. 229–254.
-
- Huang L., Pookanjanatavip,M., Gu,X. and Santi,D.V. (1998) Biochemistry, 37, 344–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

