Applications of flow cytometry to clinical microbiology
- PMID: 10755996
- PMCID: PMC100149
- DOI: 10.1128/CMR.13.2.167
Applications of flow cytometry to clinical microbiology
Abstract
Classical microbiology techniques are relatively slow in comparison to other analytical techniques, in many cases due to the need to culture the microorganisms. Furthermore, classical approaches are difficult with unculturable microorganisms. More recently, the emergence of molecular biology techniques, particularly those on antibodies and nucleic acid probes combined with amplification techniques, has provided speediness and specificity to microbiological diagnosis. Flow cytometry (FCM) allows single- or multiple-microbe detection in clinical samples in an easy, reliable, and fast way. Microbes can be identified on the basis of their peculiar cytometric parameters or by means of certain fluorochromes that can be used either independently or bound to specific antibodies or oligonucleotides. FCM has permitted the development of quantitative procedures to assess antimicrobial susceptibility and drug cytotoxicity in a rapid, accurate, and highly reproducible way. Furthermore, this technique allows the monitoring of in vitro antimicrobial activity and of antimicrobial treatments ex vivo. The most outstanding contribution of FCM is the possibility of detecting the presence of heterogeneous populations with different responses to antimicrobial treatments. Despite these advantages, the application of FCM in clinical microbiology is not yet widespread, probably due to the lack of access to flow cytometers or the lack of knowledge about the potential of this technique. One of the goals of this review is to attempt to mitigate this latter circumstance. We are convinced that in the near future, the availability of commercial kits should increase the use of this technique in the clinical microbiology laboratory.
Figures
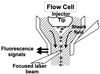
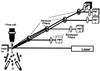
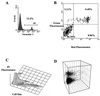
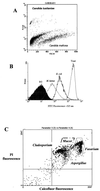

Similar articles
-
Microbiology.Clin Lab Med. 2001 Dec;21(4):897-909, x-xi. Clin Lab Med. 2001. PMID: 11770294 Review.
-
Fluorescence staining and flow cytometry for monitoring microbial cells.J Immunol Methods. 2000 Sep 21;243(1-2):191-210. doi: 10.1016/s0022-1759(00)00234-9. J Immunol Methods. 2000. PMID: 10986415 Review.
-
Overview of Flow Cytometry and Microbiology.Curr Protoc Cytom. 2018 Apr;84(1):e37. doi: 10.1002/cpcy.37. Curr Protoc Cytom. 2018. PMID: 30040224 Review.
-
Application of flow cytometry to wine microorganisms.Food Microbiol. 2017 Apr;62:221-231. doi: 10.1016/j.fm.2016.10.023. Epub 2016 Oct 18. Food Microbiol. 2017. PMID: 27889152 Review.
-
Detection of specific microorganisms in environmental samples using flow cytometry.Methods Cell Biol. 1994;42 Pt B:489-522. doi: 10.1016/s0091-679x(08)61092-4. Methods Cell Biol. 1994. PMID: 7533254 No abstract available.
Cited by
-
New approach for serological testing for leptospirosis by using detection of leptospira agglutination by flow cytometry light scatter analysis.J Clin Microbiol. 2004 Apr;42(4):1680-5. doi: 10.1128/JCM.42.4.1680-1685.2004. J Clin Microbiol. 2004. PMID: 15071025 Free PMC article.
-
A Technique for Rapid Bacterial-Density Enumeration through Membrane Filtration and Differential Pressure Measurements.Micromachines (Basel). 2022 Jul 28;13(8):1198. doi: 10.3390/mi13081198. Micromachines (Basel). 2022. PMID: 36014121 Free PMC article.
-
A streamlined workflow for a fast and cost-effective count of tyndallized probiotics using flow cytometry.Front Microbiol. 2024 May 3;15:1389069. doi: 10.3389/fmicb.2024.1389069. eCollection 2024. Front Microbiol. 2024. PMID: 38765688 Free PMC article.
-
Ligand engagement of Toll-like receptors regulates their expression in cortical microglia and astrocytes.J Neuroinflammation. 2015 Dec 30;12:244. doi: 10.1186/s12974-015-0458-6. J Neuroinflammation. 2015. PMID: 26714634 Free PMC article.
-
Antifungal susceptibility testing: practical aspects and current challenges.Clin Microbiol Rev. 2001 Oct;14(4):643-58, table of contents. doi: 10.1128/CMR.14.4.643-658.2001. Clin Microbiol Rev. 2001. PMID: 11585779 Free PMC article. Review.
References
-
- Reference deleted.
-
- Agar D W, Bailey J E. Cell cycle operation during batch growth of fission yeast populations. Cytometry. 1982;3:123–128. - PubMed
-
- Akbar A N, Borthwick N, Salmon M, Gombert W, Bofill M, Shamsadeen N, Pilling D, Pett S, Grundy J E, Janossy G. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993;178:427–438. - PMC - PubMed
-
- Alberghina L, Porro D. Quantitative flow cytometry: analysis of protein distributions in budding yeast. A mini-review. Yeast. 1993;9:815–823. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

