Calreticulin couples calcium release and calcium influx in integrin-mediated calcium signaling
- PMID: 10749940
- PMCID: PMC14857
- DOI: 10.1091/mbc.11.4.1433
Calreticulin couples calcium release and calcium influx in integrin-mediated calcium signaling
Abstract
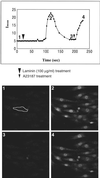
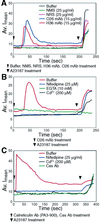
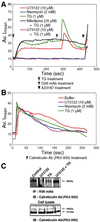
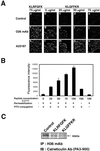
The engagement of integrin alpha7 in E63 skeletal muscle cells by laminin or anti-alpha7 antibodies triggered transient elevations in the intracellular free Ca(2+) concentration that resulted from both inositol triphosphate-evoked Ca(2+) release from intracellular stores and extracellular Ca(2+) influx through voltage-gated, L-type Ca(2+) channels. The extracellular domain of integrin alpha7 was found to associate with both ectocalreticulin and dihydropyridine receptor on the cell surface. Calreticulin appears to also associate with cytoplasmic domain of integrin alpha7 in a manner highly dependent on the cytosolic Ca(2+) concentration. It appeared that intracellular Ca(2+) release was a prerequisite for Ca(2+) influx and that calreticulin associated with the integrin cytoplasmic domain mediated the coupling of between the Ca(2+) release and Ca(2+) influx. These findings suggest that calreticulin serves as a cytosolic activator of integrin and a signal transducer between integrins and Ca(2+) channels on the cell surface.
Figures

Similar articles
-
Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion.Nature. 1997 Apr 24;386(6627):843-7. doi: 10.1038/386843a0. Nature. 1997. PMID: 9126744
-
Calreticulin modulates capacitative Ca2+ influx by controlling the extent of inositol 1,4,5-trisphosphate-induced Ca2+ store depletion.J Biol Chem. 2000 Nov 24;275(47):36676-82. doi: 10.1074/jbc.M002041200. J Biol Chem. 2000. PMID: 10973951
-
Role of calreticulin in regulating intracellular Ca2+ storage and capacitative Ca2+ entry in HeLa cells.Cell Calcium. 1998 Oct;24(4):253-62. doi: 10.1016/s0143-4160(98)90049-5. Cell Calcium. 1998. PMID: 9883279
-
Metabotropic Ca2+ channel-induced calcium release in vascular smooth muscle.Cell Calcium. 2007 Oct-Nov;42(4-5):513-20. doi: 10.1016/j.ceca.2007.04.010. Epub 2007 Jun 7. Cell Calcium. 2007. PMID: 17559931 Review.
-
Abnormal intracellular ca(2+)homeostasis and disease.Cell Calcium. 2000 Jul;28(1):1-21. doi: 10.1054/ceca.2000.0131. Cell Calcium. 2000. PMID: 10942700 Review.
Cited by
-
The membrane-proximal KXGFFKR motif of α-integrin mediates chemoresistance.Mol Cell Biol. 2013 Nov;33(21):4334-45. doi: 10.1128/MCB.00580-13. Epub 2013 Sep 3. Mol Cell Biol. 2013. PMID: 24001772 Free PMC article.
-
Epithelial tissue geometry directs emergence of bioelectric field and pattern of proliferation.Mol Biol Cell. 2020 Jul 21;31(16):1691-1702. doi: 10.1091/mbc.E19-12-0719. Epub 2020 Jun 10. Mol Biol Cell. 2020. PMID: 32520653 Free PMC article.
-
Calreticulin enhances porcine wound repair by diverse biological effects.Am J Pathol. 2008 Sep;173(3):610-30. doi: 10.2353/ajpath.2008.071027. Am J Pathol. 2008. PMID: 18753412 Free PMC article.
-
Structural insight into the role of thrombospondin-1 binding to calreticulin in calreticulin-induced focal adhesion disassembly.Biochemistry. 2010 May 4;49(17):3685-94. doi: 10.1021/bi902067f. Biochemistry. 2010. PMID: 20337411 Free PMC article.
-
Calreticulin promotes EGF-induced EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling pathway.Cell Death Dis. 2017 Oct 26;8(10):e3147. doi: 10.1038/cddis.2017.547. Cell Death Dis. 2017. PMID: 29072694 Free PMC article.
References
-
- Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–2880. - PubMed
-
- Baksh S, Michalak M. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J Biol Chem. 1991;266:21458–21465. - PubMed
-
- Bastianutto C, Clementi E, Codazzi F, Podini P, De Giorgi F, Rizzuto R, Meldolesi J, Pozzan T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J Cell Biol. 1995;130:847–855. - PMC - PubMed
-
- Bouvard D, Molla A, Block MR. Calcium/calmodulin-dependent protein kinase II controls α5β1 integrin-mediated inside-out signaling. J Cell Sci. 1998;111:657–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

