Recognition of triple-helical DNA structures by transposon Tn7
- PMID: 10737770
- PMCID: PMC18120
- DOI: 10.1073/pnas.080061497
Recognition of triple-helical DNA structures by transposon Tn7
Abstract
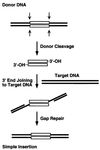
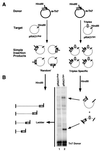
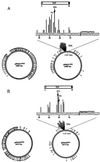
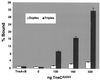
We have found that the bacterial transposon Tn7 can recognize and preferentially insert adjacent to triple-helical nucleic acid structures. Both synthetic intermolecular triplexes, formed through the pairing of a short triplex-forming oligonucleotide on a plasmid DNA, and naturally occurring mirror repeat sequences known to form intramolecular triplexes or H-form DNA are preferential targets for Tn7 insertion in vitro. This target site selectivity depends upon the recognition of the triplex region by a Tn7-encoded ATP-using protein, TnsC, which controls Tn7 target site selection: the interaction of TnsC with the triplex region results in recruitment and activation of the Tn7 transposase. Recognition of a nucleic acid structural motif provides both new information into the factors that influence Tn7's target site selection and broadens its targeting capabilities.
Figures


Similar articles
-
Target DNA structure plays a critical role in Tn7 transposition.EMBO J. 2001 Feb 15;20(4):924-32. doi: 10.1093/emboj/20.4.924. EMBO J. 2001. PMID: 11179236 Free PMC article.
-
Selective recognition of pyrimidine motif triplexes by a protein encoded by the bacterial transposon Tn7.J Mol Biol. 2001 Apr 13;307(5):1161-70. doi: 10.1006/jmbi.2001.4553. J Mol Biol. 2001. PMID: 11292332
-
A minimal system for Tn7 transposition: the transposon-encoded proteins TnsA and TnsB can execute DNA breakage and joining reactions that generate circularized Tn7 species.J Mol Biol. 2000 Mar 17;297(1):25-37. doi: 10.1006/jmbi.2000.3558. J Mol Biol. 2000. PMID: 10704304
-
Tn7.Microbiol Spectr. 2014 Oct;2(5). doi: 10.1128/microbiolspec.MDNA3-0010-2014. Microbiol Spectr. 2014. PMID: 26104363 Review.
-
DNA triple helices: biological consequences and therapeutic potential.Biochimie. 2008 Aug;90(8):1117-30. doi: 10.1016/j.biochi.2008.02.011. Epub 2008 Feb 21. Biochimie. 2008. PMID: 18331847 Free PMC article. Review.
Cited by
-
Architecture of the Tn7 posttransposition complex: an elaborate nucleoprotein structure.J Mol Biol. 2010 Aug 13;401(2):167-81. doi: 10.1016/j.jmb.2010.06.003. Epub 2010 Jun 9. J Mol Biol. 2010. PMID: 20538004 Free PMC article.
-
Conformational toggling controls target site choice for the heteromeric transposase element Tn7.Nucleic Acids Res. 2015 Dec 15;43(22):10734-45. doi: 10.1093/nar/gkv913. Epub 2015 Sep 17. Nucleic Acids Res. 2015. PMID: 26384427 Free PMC article.
-
Transposon Tn7 preferentially inserts into GAA*TTC triplet repeats under conditions conducive to Y*R*Y triplex formation.PLoS One. 2010 Jun 15;5(6):e11121. doi: 10.1371/journal.pone.0011121. PLoS One. 2010. PMID: 20559546 Free PMC article.
-
Target DNA structure plays a critical role in Tn7 transposition.EMBO J. 2001 Feb 15;20(4):924-32. doi: 10.1093/emboj/20.4.924. EMBO J. 2001. PMID: 11179236 Free PMC article.
-
Cargo Genes of Tn7-Like Transposons Comprise an Enormous Diversity of Defense Systems, Mobile Genetic Elements, and Antibiotic Resistance Genes.mBio. 2021 Dec 21;12(6):e0293821. doi: 10.1128/mBio.02938-21. Epub 2021 Dec 7. mBio. 2021. PMID: 34872347 Free PMC article.
References
-
- Berg D E, Howe M M, editors. Mobile DNA. Washington, DC: Am. Soc. Microbiol.; 1989.
-
- Craig N L. Annu Rev Biochem. 1997;66:437–474. - PubMed
-
- Berg C M, Berg E. In: Mobile Genetic Elements. Sherratt D J, editor. Oxford: IRL; 1995. pp. 38–68.
-
- Katz R A, Gravuer K, Skalka A M. J Biol Chem. 1998;273:24190–24195. - PubMed
-
- Pryciak P M, Varmus H E. Cell. 1992;69:769–780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

