Proteomics of the chloroplast: systematic identification and targeting analysis of lumenal and peripheral thylakoid proteins
- PMID: 10715320
- PMCID: PMC139834
- DOI: 10.1105/tpc.12.3.319
Proteomics of the chloroplast: systematic identification and targeting analysis of lumenal and peripheral thylakoid proteins
Abstract
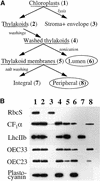
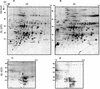
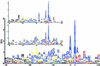
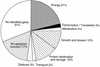
The soluble and peripheral proteins in the thylakoids of pea were systematically analyzed by using two-dimensional electrophoresis, mass spectrometry, and N-terminal Edman sequencing, followed by database searching. After correcting to eliminate possible isoforms and post-translational modifications, we estimated that there are at least 200 to 230 different lumenal and peripheral proteins. Sixty-one proteins were identified; for 33 of these proteins, a clear function or functional domain could be identified, whereas for 10 proteins, no function could be assigned. For 18 proteins, no expressed sequence tag or full-length gene could be identified in the databases, despite experimental determination of a significant amount of amino acid sequence. Nine previously unidentified proteins with lumenal transit peptides are presented along with their full-length genes; seven of these proteins possess the twin arginine motif that is characteristic for substrates of the TAT pathway. Logoplots were used to provide a detailed analysis of the lumenal targeting signals, and all nuclear-encoded proteins identified on the two-dimensional gels were used to test predictions for chloroplast localization and transit peptides made by the software programs ChloroP, PSORT, and SignalP. A combination of these three programs was found to provide a useful tool for evaluating chloroplast localization and transit peptides and also could reveal possible alternative processing sites and dual targeting. The potential of proteomics for plant biology and homology-based searching with mass spectrometry data is discussed.
Figures



Comment in
-
Proteomics: broad strokes of expressionism?Plant Cell. 2000 Mar;12(3):303-4. doi: 10.1105/tpc.12.3.303. Plant Cell. 2000. PMID: 10715314 Free PMC article. No abstract available.
Similar articles
-
Central functions of the lumenal and peripheral thylakoid proteome of Arabidopsis determined by experimentation and genome-wide prediction.Plant Cell. 2002 Jan;14(1):211-36. doi: 10.1105/tpc.010304. Plant Cell. 2002. PMID: 11826309 Free PMC article.
-
PSII-T, a new nuclear encoded lumenal protein from photosystem II. Targeting and processing in isolated chloroplasts.J Biol Chem. 1995 May 19;270(20):12197-202. doi: 10.1074/jbc.270.20.12197. J Biol Chem. 1995. PMID: 7744870
-
The ten amino acids of the oxygen-evolving enhancer of tobacco is sufficient as the peptide residues for protein transport to the chloroplast thylakoid.Plant Mol Biol. 2021 Mar;105(4-5):513-523. doi: 10.1007/s11103-020-01106-8. Epub 2021 Jan 3. Plant Mol Biol. 2021. PMID: 33393067 Free PMC article.
-
Mechanisms of protein import into thylakoids of chloroplasts.Biol Chem. 2007 Sep;388(9):907-15. doi: 10.1515/BC.2007.111. Biol Chem. 2007. PMID: 17696774 Review.
-
Targeting of lumenal proteins across the thylakoid membrane.J Exp Bot. 2012 Feb;63(4):1689-98. doi: 10.1093/jxb/err444. Epub 2012 Jan 24. J Exp Bot. 2012. PMID: 22275386 Review.
Cited by
-
An efficient proteomic approach to analyze agriculture crop biomass.Protein J. 2013 Jun;32(5):365-72. doi: 10.1007/s10930-013-9495-0. Protein J. 2013. PMID: 23681363
-
Expression of the minor isoform pea ferredoxin in tobacco alters photosynthetic electron partitioning and enhances cyclic electron flow.Plant Physiol. 2013 Feb;161(2):866-79. doi: 10.1104/pp.112.211078. Epub 2012 Dec 12. Plant Physiol. 2013. PMID: 23370717 Free PMC article.
-
Secretome analysis reveals an Arabidopsis lipase involved in defense against Alternaria brassicicola.Plant Cell. 2005 Oct;17(10):2832-47. doi: 10.1105/tpc.105.034819. Epub 2005 Aug 26. Plant Cell. 2005. PMID: 16126835 Free PMC article.
-
Proteomics and a future generation of plant molecular biologists.Plant Mol Biol. 2002 Jan;48(1-2):143-54. Plant Mol Biol. 2002. PMID: 11862971 Review.
-
Chloroplast and mitochondrial proteases in Arabidopsis. A proposed nomenclature.Plant Physiol. 2001 Apr;125(4):1912-8. doi: 10.1104/pp.125.4.1912. Plant Physiol. 2001. PMID: 11299370 Free PMC article.
References
-
- Adam, Z. (1996). Protein stability and degradation in chloroplasts. Plant Mol. Biol. 32 773–783. - PubMed
-
- Bauw, G., De Loose, M., Inzé, D., van Montagu, M., and Vandekerckhove, J. (1987). Alterations in the phenotype of plant cells studied by NH2-terminal amino acid–sequence analysis of proteins electroblotted from two-dimensional gel-separated total extracts. Proc. Natl. Acad. Sci. USA 84 4806–4810. - PMC - PubMed
-
- Belanger, F.C., Leustek, T., Chu, B., and Kriz, A.L. (1995). Evidence for the thiamine biosynthetic pathway in higher-plant plastids and its developmental regulation. Plant Mol. Biol. 29 809–821. - PubMed
-
- Bevan, M., et al., (1998). Analysis of 1.9 Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana. Nature 39 485–488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

