Initiation of translation by non-AUG codons in human T-cell lymphotropic virus type I mRNA encoding both Rex and Tax regulatory proteins
- PMID: 10710429
- PMCID: PMC102795
- DOI: 10.1093/nar/28.7.1625
Initiation of translation by non-AUG codons in human T-cell lymphotropic virus type I mRNA encoding both Rex and Tax regulatory proteins
Abstract
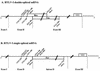
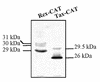
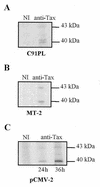
Human T-cell lymphotropic virus type I (HTLV-I) double-spliced mRNA exhibits two GUG and two CUG codons upstream to, and in frame with, the sequences encoding Rex and Tax regulatory proteins, respectively. To verify whether these GUG and CUG codons could be used as additional initiation codons of translation, two chimeric constructs were built for directing the synthesis of either Rex-CAT or Tax-CAT fusion proteins. In both cases, the CAT reporter sequence was inserted after the Tax AUG codon and in frame with either the Rex or Tax AUG codon. Under transient expression of these constructs, other proteins of higher molecular mass were synthesized in addition to the expected Rex-CAT and Tax-CAT proteins. The potential non-AUG initiation codons were exchanged for either an AUG codon or a non-initiation codon. This allowed us to demonstrate that the two GUG codons in frame with the Rex coding sequence, and only the second CUG in frame with the Tax coding sequence, were used as additional initiation codons. In HTLV-I infected cells, two Rex and one Tax additional proteins were detected that exhibited molecular mass compatible with the use of the two GUG and the second CUG as additional initiation codons of translation. Comparison of the HTLV-I proviral DNA sequence with that of other HTLV-related retroviruses revealed a striking conservation of the three non-AUG initiation codons, strongly suggesting their use for the synthesis of additional Rex and Tax proteins.
Figures



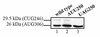

Similar articles
-
Up-regulation of human T lymphotropic virus type 1 (HTLV-1) tax/rex mRNA in infected lung tissues.Clin Exp Immunol. 2000 Jun;120(3):488-98. doi: 10.1046/j.1365-2249.2000.01237.x. Clin Exp Immunol. 2000. PMID: 10844528 Free PMC article.
-
Nuclear export and expression of human T-cell leukemia virus type 1 tax/rex mRNA are RxRE/Rex dependent.J Virol. 2012 Apr;86(8):4559-65. doi: 10.1128/JVI.06361-11. Epub 2012 Feb 8. J Virol. 2012. PMID: 22318152 Free PMC article.
-
The genomic structure of a new simian T-lymphotropic virus, STLV-PH969, differs from that of human T-lymphotropic virus types I and II.J Gen Virol. 1996 Feb;77 ( Pt 2 ):347-58. doi: 10.1099/0022-1317-77-2-347. J Gen Virol. 1996. PMID: 8627239
-
HTLV-1 Yin and Yang: Rex and p30 master regulators of viral mRNA trafficking.AIDS Rev. 2008 Oct-Dec;10(4):195-204. AIDS Rev. 2008. PMID: 19092975 Free PMC article. Review.
-
Tuning Rex rules HTLV-1 pathogenesis.Front Immunol. 2022 Sep 16;13:959962. doi: 10.3389/fimmu.2022.959962. eCollection 2022. Front Immunol. 2022. PMID: 36189216 Free PMC article. Review.
Cited by
-
Comprehensive annotation of Glossina pallidipes salivary gland hypertrophy virus from Ethiopian tsetse flies: a proteogenomics approach.J Gen Virol. 2016 Apr;97(4):1010-1031. doi: 10.1099/jgv.0.000409. Epub 2016 Jan 21. J Gen Virol. 2016. PMID: 26801744 Free PMC article.
-
Accessory protein 5a is a major antagonist of the antiviral action of interferon against murine coronavirus.J Virol. 2010 Aug;84(16):8262-74. doi: 10.1128/JVI.00385-10. Epub 2010 Jun 2. J Virol. 2010. PMID: 20519394 Free PMC article.
-
Virus versus host cell translation love and hate stories.Adv Virus Res. 2009;73:99-170. doi: 10.1016/S0065-3527(09)73003-9. Adv Virus Res. 2009. PMID: 19695382 Free PMC article. Review.
-
Human Immunodeficiency Virus-Type 1 LTR DNA contains an intrinsic gene producing antisense RNA and protein products.Retrovirology. 2006 Nov 8;3:80. doi: 10.1186/1742-4690-3-80. Retrovirology. 2006. PMID: 17090330 Free PMC article.
-
Unconventional viral gene expression mechanisms as therapeutic targets.Nature. 2021 May;593(7859):362-371. doi: 10.1038/s41586-021-03511-5. Epub 2021 May 19. Nature. 2021. PMID: 34012080 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

