Covalent modification of the transactivator protein IE2-p86 of human cytomegalovirus by conjugation to the ubiquitin-homologous proteins SUMO-1 and hSMT3b
- PMID: 10684265
- PMCID: PMC111739
- DOI: 10.1128/jvi.74.6.2510-2524.2000
Covalent modification of the transactivator protein IE2-p86 of human cytomegalovirus by conjugation to the ubiquitin-homologous proteins SUMO-1 and hSMT3b
Abstract
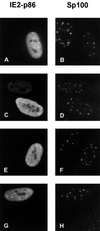
The 86-kDa IE2 protein (IE2-p86) of human cytomegalovirus (HCMV) is a potent transactivator of viral as well as cellular promoters. Several lines of evidence indicate that this broad transactivation spectrum is mediated by protein-protein interactions. To identify novel cellular binding partners, we performed a yeast two-hybrid screen using a N-terminal deletion mutant of IE2-p86 comprising amino acids 135 to 579 as a bait. Here, we report the isolation of two ubiquitin-homologous proteins, SUMO-1 and hSMT3b, as well as their conjugating activity hUBC9 (human ubiquitin-conjugating enzyme 9) as specific interaction partners of HCMV IE2. The polypeptides SUMO-1 and hSMT3b have previously been shown to be covalently coupled to a subset of nuclear proteins such as the nuclear domain 10 (ND10) proteins PML and Sp100 in a manner analogous to ubiquitinylation, which we call SUMOylation. By Western blot analysis, we were able to show that the IE2-p86 protein can be partially converted to a 105-kDa isoform in a dose-dependent manner after cotransfection of an epitope-tagged SUMO-1. Immunoprecipitation experiments of the conjugated isoforms using denaturing conditions further confirmed the covalent coupling of SUMO-1 or hSMT3b to IE2-p86 both after transient transfection and after lytic infection of human primary fibroblasts. Moreover, we defined two modification sites within IE2, located in an immediate vicinity at amino acid positions 175 and 180, which appear to be used alternatively for coupling. By using a SUMOylation-defective mutant, we showed that the targeting of IE2-p86 to ND10 occurs independent of this modification. However, a strong reduction of IE2-mediated transactivation of two viral early promoters and a heterologous promoter was observed in cotransfection analysis with the SUMOylation-defective mutant. This suggests a functional relevance of covalent modification by ubiquitin-homologous proteins for IE2-mediated transactivation, possibly by providing an additional interaction motif for cellular cofactors.
Figures

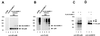
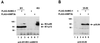



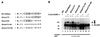


Similar articles
-
Evaluation of interactions of human cytomegalovirus immediate-early IE2 regulatory protein with small ubiquitin-like modifiers and their conjugation enzyme Ubc9.J Virol. 2001 Apr;75(8):3859-72. doi: 10.1128/JVI.75.8.3859-3872.2001. J Virol. 2001. PMID: 11264375 Free PMC article.
-
The ND10 Component Promyelocytic Leukemia Protein Acts as an E3 Ligase for SUMOylation of the Major Immediate Early Protein IE1 of Human Cytomegalovirus.J Virol. 2017 Apr 28;91(10):e02335-16. doi: 10.1128/JVI.02335-16. Print 2017 May 15. J Virol. 2017. PMID: 28250117 Free PMC article.
-
The UL84 protein of human cytomegalovirus acts as a transdominant inhibitor of immediate-early-mediated transactivation that is able to prevent viral replication.J Virol. 1997 Sep;71(9):7048-60. doi: 10.1128/JVI.71.9.7048-7060.1997. J Virol. 1997. PMID: 9261435 Free PMC article.
-
Intracellular targeting of proteins by sumoylation.Exp Cell Res. 2001 Nov 15;271(1):57-65. doi: 10.1006/excr.2001.5366. Exp Cell Res. 2001. PMID: 11697882 Review.
-
New insights into the role of the small ubiquitin-like modifier (SUMO) in plants.Int Rev Cell Mol Biol. 2013;300:161-209. doi: 10.1016/B978-0-12-405210-9.00005-9. Int Rev Cell Mol Biol. 2013. PMID: 23273862 Review.
Cited by
-
Proteasome-independent disruption of PML oncogenic domains (PODs), but not covalent modification by SUMO-1, is required for human cytomegalovirus immediate-early protein IE1 to inhibit PML-mediated transcriptional repression.J Virol. 2001 Nov;75(22):10683-95. doi: 10.1128/JVI.75.22.10683-10695.2001. J Virol. 2001. PMID: 11602710 Free PMC article.
-
A novel transferable nuclear export signal mediates CRM1-independent nucleocytoplasmic shuttling of the human cytomegalovirus transactivator protein pUL69.EMBO J. 2001 Dec 17;20(24):7271-83. doi: 10.1093/emboj/20.24.7271. EMBO J. 2001. PMID: 11743003 Free PMC article.
-
SUMO-1 modification of human cytomegalovirus IE1/IE72.J Virol. 2002 Mar;76(6):2990-6. doi: 10.1128/jvi.76.6.2990-2996.2002. J Virol. 2002. PMID: 11861864 Free PMC article.
-
Dual signaling via interferon and DNA damage response elicits entrapment by giant PML nuclear bodies.Elife. 2022 Mar 23;11:e73006. doi: 10.7554/eLife.73006. Elife. 2022. PMID: 35319461 Free PMC article.
-
Cross-Species Analysis of Innate Immune Antagonism by Cytomegalovirus IE1 Protein.Viruses. 2022 Jul 26;14(8):1626. doi: 10.3390/v14081626. Viruses. 2022. PMID: 35893691 Free PMC article.
References
-
- Alford C. Breast milk transmission of cytomegalovirus (CMV) infection. Adv Exp Med Biol. 1991;310:293–299. - PubMed
-
- Alford C A, Britt W J. Cytomegalovirus. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press, Ltd.; 1990. pp. 1981–2010.
-
- Andreoni M, Faircloth M, Vugler L, Britt W J. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J Virol Methods. 1989;23:157–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

