Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts
- PMID: 10683372
- PMCID: PMC289172
- DOI: 10.1172/JCI8905
Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts
Abstract
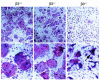
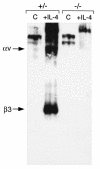
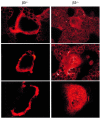
Osteoclasts express the alphavbeta3 integrin, an adhesion receptor that has been implicated in bone resorption and that is therefore a potential therapeutic target. To assess the role of this heterodimer in skeletal development in vivo, we engineered mice in which the gene for the beta3 integrin subunit was deleted. Bone marrow macrophages derived from these mutants differentiate in vitro into numerous osteoclasts, thus establishing that alphavbeta3 is not necessary for osteoclast recruitment. Furthermore, the closely related integrin, alphavbeta5, does not substitute for alphavbeta3 during cytokine stimulation or authentic osteoclastogenesis. beta3 knockout mice, but not their heterozygous littermates, develop histologically and radiographically evident osteosclerosis with age. Despite their increased bone mass, beta3-null mice contain 3.5-fold more osteoclasts than do heterozygotes. These mutant osteoclasts are, however, dysfunctional, as evidenced by their reduced ability to resorb whale dentin in vitro and the significant hypocalcemia seen in the knockout mice. The resorptive defect in beta3-deficient osteoclasts may reflect absence of matrix-derived intracellular signals, since their cytoskeleton is distinctly abnormal and they fail to spread in vitro, to form actin rings ex vivo, or to form normal ruffled membranes in vivo. Thus, although it is not required for osteoclastogenesis, the integrin alphavbeta3 is essential for normal osteoclast function.
Figures







Similar articles
-
Critical role of beta3 integrin in experimental postmenopausal osteoporosis.J Bone Miner Res. 2005 Dec;20(12):2116-23. doi: 10.1359/JBMR.050724. Epub 2005 Jul 25. J Bone Miner Res. 2005. PMID: 16294265
-
Mice lacking the integrin beta5 subunit have accelerated osteoclast maturation and increased activity in the estrogen-deficient state.J Bone Miner Res. 2005 Jan;20(1):58-66. doi: 10.1359/JBMR.041017. Epub 2004 Oct 25. J Bone Miner Res. 2005. PMID: 15619670
-
A Glanzmann's mutation in beta 3 integrin specifically impairs osteoclast function.J Clin Invest. 2001 May;107(9):1137-44. doi: 10.1172/JCI12040. J Clin Invest. 2001. PMID: 11342577 Free PMC article.
-
Regulatory mechanism of osteoclast activation.J Electron Microsc (Tokyo). 2003;52(6):527-33. doi: 10.1093/jmicro/52.6.527. J Electron Microsc (Tokyo). 2003. PMID: 14756240 Review.
-
Integrin-mediated signaling in the regulation of osteoclast adhesion and activation.Front Biosci. 1998 Aug 1;3:d757-68. doi: 10.2741/A319. Front Biosci. 1998. PMID: 9682033 Review.
Cited by
-
Role of OSCAR Signaling in Osteoclastogenesis and Bone Disease.Front Cell Dev Biol. 2021 Apr 12;9:641162. doi: 10.3389/fcell.2021.641162. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33912557 Free PMC article. Review.
-
The biology of extracellular vesicles with focus on platelet microparticles and their role in cancer development and progression.Tumour Biol. 2016 Nov;37(11):14391-14401. doi: 10.1007/s13277-016-5358-6. Epub 2016 Sep 15. Tumour Biol. 2016. PMID: 27629289 Free PMC article. Review.
-
Rac deletion in osteoclasts causes severe osteopetrosis.J Cell Sci. 2011 Nov 15;124(Pt 22):3811-21. doi: 10.1242/jcs.086280. Epub 2011 Nov 23. J Cell Sci. 2011. PMID: 22114304 Free PMC article.
-
SLC4A2-mediated Cl-/HCO3- exchange activity is essential for calpain-dependent regulation of the actin cytoskeleton in osteoclasts.Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):2163-8. doi: 10.1073/pnas.1206392110. Epub 2013 Jan 22. Proc Natl Acad Sci U S A. 2013. PMID: 23341620 Free PMC article.
-
Role of milk fat globule-epidermal growth factor 8 in osteoimmunology.Bonekey Rep. 2016 Jul 20;5:820. doi: 10.1038/bonekey.2016.52. eCollection 2016. Bonekey Rep. 2016. PMID: 27579162 Free PMC article. Review.
References
-
- Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. - PubMed
-
- Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245:855–857. - PubMed
-
- Horton MA, Taylor ML, Arnett TR, Helfrich MH. Arg-gly-asp (RGD) peptides and the anti-vitronectin receptor antibody 23C6 inhibit dentine resorption and cell spreading by osteoclasts. Exp Cell Res. 1991;195:368–375. - PubMed
-
- Nakamura I, Tanaka H, Rodan GA, Duong LT. Echistatin inhibits the migration of murine prefusion osteoclasts and the formation of multinucleated osteoclast-like cells. Endocrinology. 1998;139:5182–5193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

