Complex protein interactions within the human polyadenylation machinery identify a novel component
- PMID: 10669729
- PMCID: PMC85326
- DOI: 10.1128/MCB.20.5.1515-1525.2000
Complex protein interactions within the human polyadenylation machinery identify a novel component
Abstract
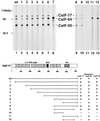
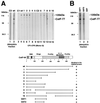
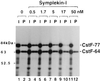
Polyadenylation of mRNA precursors is a two-step reaction requiring multiple protein factors. Cleavage stimulation factor (CstF) is a heterotrimer necessary for the first step, endonucleolytic cleavage, and it plays an important role in determining the efficiency of polyadenylation. Although a considerable amount is known about the RNA binding properties of CstF, the protein-protein interactions required for its assembly and function are poorly understood. We therefore first identified regions of the CstF subunits, CstF-77, CstF-64, and CstF-50, required for interaction with each other. Unexpectedly, small regions of two of the subunits participate in multiple interactions. In CstF-77, a proline-rich domain is necessary not only for binding both other subunits but also for self-association, an interaction consistent with genetic studies in Drosophila. In CstF-64, a small region, highly conserved in metazoa, is responsible for interactions with two proteins, CstF-77 and symplekin, a nuclear protein of previously unknown function. Intriguingly, symplekin has significant similarity to a yeast protein, PTA1, that is a component of the yeast polyadenylation machinery. We show that multiple factors, including CstF, cleavage-polyadenylation specificity factor, and symplekin, can be isolated from cells as part of a large complex. These and other data suggest that symplekin may function in assembly of the polyadenylation machinery.
Figures



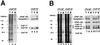
Similar articles
-
Interactions of CstF-64, CstF-77, and symplekin: implications on localisation and function.Mol Biol Cell. 2011 Jan 1;22(1):91-104. doi: 10.1091/mbc.E10-06-0543. Epub 2010 Nov 30. Mol Biol Cell. 2011. PMID: 21119002 Free PMC article.
-
The Drosophila homologue of the 64 kDa subunit of cleavage stimulation factor interacts with the 77 kDa subunit encoded by the suppressor of forked gene.Nucleic Acids Res. 2000 Jan 15;28(2):520-6. doi: 10.1093/nar/28.2.520. Nucleic Acids Res. 2000. PMID: 10606651 Free PMC article.
-
Cloning and characterization of Arabidopsis homologues of the animal CstF complex that regulates 3' mRNA cleavage and polyadenylation.J Exp Bot. 2002 Nov;53(378):2277-8. doi: 10.1093/jxb/erf073. J Exp Bot. 2002. PMID: 12379796
-
Structural biology of poly(A) site definition.Wiley Interdiscip Rev RNA. 2011 Sep-Oct;2(5):732-47. doi: 10.1002/wrna.88. Epub 2011 Apr 27. Wiley Interdiscip Rev RNA. 2011. PMID: 21823232 Free PMC article. Review.
-
A history of poly A sequences: from formation to factors to function.Prog Nucleic Acid Res Mol Biol. 2002;71:285-389. doi: 10.1016/s0079-6603(02)71046-5. Prog Nucleic Acid Res Mol Biol. 2002. PMID: 12102557 Review.
Cited by
-
Localization of RNAPII and 3' end formation factor CstF subunits on C. elegans genes and operons.Transcription. 2016 May 26;7(3):96-110. doi: 10.1080/21541264.2016.1168509. Epub 2016 Apr 28. Transcription. 2016. PMID: 27124504 Free PMC article.
-
Polyadenylation proteins CstF-64 and tauCstF-64 exhibit differential binding affinities for RNA polymers.Biochem J. 2007 Feb 1;401(3):651-8. doi: 10.1042/BJ20061097. Biochem J. 2007. PMID: 17029590 Free PMC article.
-
Sumoylation modulates the assembly and activity of the pre-mRNA 3' processing complex.Mol Cell Biol. 2007 Dec;27(24):8848-58. doi: 10.1128/MCB.01186-07. Epub 2007 Oct 8. Mol Cell Biol. 2007. PMID: 17923699 Free PMC article.
-
Prediction of the archaeal exosome and its connections with the proteasome and the translation and transcription machineries by a comparative-genomic approach.Genome Res. 2001 Feb;11(2):240-52. doi: 10.1101/gr.162001. Genome Res. 2001. PMID: 11157787 Free PMC article.
-
Intercistronic region required for polycistronic pre-mRNA processing in Caenorhabditis elegans.Mol Cell Biol. 2001 Feb;21(4):1111-20. doi: 10.1128/MCB.21.4.1111-1120.2001. Mol Cell Biol. 2001. PMID: 11158298 Free PMC article.
References
-
- Beyer K, Dandekar T, Keller W. RNA ligands selected by cleavage stimulation factor contain distinct sequence motifs that function as downstream elements in 3′-end processing of pre-mRNA. J Biol Chem. 1997;272:26769–26779. - PubMed
-
- Colgan D F, Manley J L. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 1997;11:2755–2766. - PubMed
-
- Dantonel J-C, Murthy K G K, Manley J L, Tora L. CPSF links transcription and mRNA 3′ end formation. Nature. 1997;389:399–402. - PubMed
-
- Faber P W, Barnes G T, Srinidhi J, Chen J, Gusella J F, MacDonald M E. Huntingtin interacts with a family of WW domain proteins. Hum Mol Genet. 1998;7:1463–1474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
