Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization
- PMID: 10648569
- PMCID: PMC2174278
- DOI: 10.1083/jcb.148.2.363
Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization
Abstract
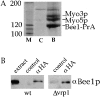
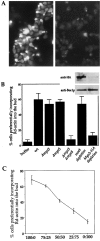
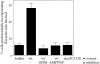
The generation of cortical actin filaments is necessary for processes such as cell motility and cell polarization. Several recent studies have demonstrated that Wiskott-Aldrich syndrome protein (WASP) family proteins and the actin-related protein (Arp) 2/3 complex are key factors in the nucleation of actin filaments in diverse eukaryotic organisms. To identify other factors involved in this process, we have isolated proteins that bind to Bee1p/Las17p, the yeast WASP-like protein, by affinity chromatography and mass spectroscopic analysis. The yeast type I myosins, Myo3p and Myo5p, have both been identified as Bee1p-interacting proteins. Like Bee1p, these myosins are essential for cortical actin assembly as assayed by in vitro reconstitution of actin nucleation sites in permeabilized yeast cells. Analysis using this assay further demonstrated that the motor activity of these myosins is required for the polymerization step, and that actin polymerization depends on phosphorylation of myosin motor domain by p21-activated kinases (PAKs), downstream effectors of the small guanosine triphosphatase, Cdc42p. The type I myosins also interact with the Arp2/3 complex through a sequence at the end of the tail domain homologous to the Arp2/3-activating region of WASP-like proteins. Combined deletions of the Arp2/3-interacting domains of Bee1p and the type I myosins abolish actin nucleation sites at the cortex, suggesting that these proteins function redundantly in the activation of the Arp2/3 complex.
Figures
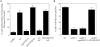


Comment in
-
The tails of two myosins.J Cell Biol. 2000 Jan 24;148(2):219-21. doi: 10.1083/jcb.148.2.219. J Cell Biol. 2000. PMID: 10648552 Free PMC article. No abstract available.
Similar articles
-
A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex.J Cell Biol. 2000 Jan 24;148(2):353-62. doi: 10.1083/jcb.148.2.353. J Cell Biol. 2000. PMID: 10648568 Free PMC article.
-
A two-tiered mechanism by which Cdc42 controls the localization and activation of an Arp2/3-activating motor complex in yeast.J Cell Biol. 2001 Oct 15;155(2):261-70. doi: 10.1083/jcb.200104094. Epub 2001 Oct 15. J Cell Biol. 2001. PMID: 11604421 Free PMC article.
-
Saccharomyces cerevisiae Bzz1p is implicated with type I myosins in actin patch polarization and is able to recruit actin-polymerizing machinery in vitro.Mol Cell Biol. 2002 Nov;22(22):7889-906. doi: 10.1128/MCB.22.22.7889-7906.2002. Mol Cell Biol. 2002. PMID: 12391157 Free PMC article.
-
Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins.Annu Rev Biochem. 2001;70:649-76. doi: 10.1146/annurev.biochem.70.1.649. Annu Rev Biochem. 2001. PMID: 11395419 Review.
-
Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins.J Biol Chem. 1999 Nov 12;274(46):32531-4. doi: 10.1074/jbc.274.46.32531. J Biol Chem. 1999. PMID: 10551802 Review. No abstract available.
Cited by
-
Widespread mRNA association with cytoskeletal motor proteins and identification and dynamics of myosin-associated mRNAs in S. cerevisiae.PLoS One. 2012;7(2):e31912. doi: 10.1371/journal.pone.0031912. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359641 Free PMC article.
-
The Arp2/3 complex promotes periodic removal of Pak1-mediated negative feedback to facilitate anticorrelated Cdc42 oscillations.bioRxiv [Preprint]. 2023 Nov 9:2023.11.08.566261. doi: 10.1101/2023.11.08.566261. bioRxiv. 2023. Update in: J Cell Biol. 2024 Sep 2;223(10):e202311139. doi: 10.1083/jcb.202311139. PMID: 38106068 Free PMC article. Updated. Preprint.
-
A Rictor-Myo1c complex participates in dynamic cortical actin events in 3T3-L1 adipocytes.Mol Cell Biol. 2008 Jul;28(13):4215-26. doi: 10.1128/MCB.00867-07. Epub 2008 Apr 21. Mol Cell Biol. 2008. PMID: 18426911 Free PMC article.
-
Distinct acto/myosin-I structures associate with endocytic profiles at the plasma membrane.J Cell Biol. 2008 Mar 24;180(6):1219-32. doi: 10.1083/jcb.200708060. Epub 2008 Mar 17. J Cell Biol. 2008. PMID: 18347067 Free PMC article.
-
Rsp5p, a new link between the actin cytoskeleton and endocytosis in the yeast Saccharomyces cerevisiae.Mol Cell Biol. 2002 Oct;22(20):6946-8. doi: 10.1128/MCB.22.20.6946-6958.2002. Mol Cell Biol. 2002. PMID: 12242276 Free PMC article.
References
-
- Adams R.J., Pollard T.D. Propulsion of organelles isolated from Acanthamoeba along actin filaments by myosin-I. Nature. 1986;322:754–756 . - PubMed
-
- Adams R.J., Pollard T.D. Binding of myosin I to membrane lipids. Nature. 1989;340:565–568 . - PubMed
-
- Aspenstrom P., Lindberg U., Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr. Biol. 1996;6:70–75 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

