Urease as a virulence factor in experimental cryptococcosis
- PMID: 10639402
- PMCID: PMC97161
- DOI: 10.1128/IAI.68.2.443-448.2000
Urease as a virulence factor in experimental cryptococcosis
Abstract
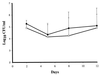
Urease catalyzes the hydrolysis of urea to ammonia and carbamate and has been found to be an important pathogenic factor for certain bacteria. Cryptococcus neoformans is a significant human pathogenic fungus that produces large amounts of urease; thus we wanted to investigate the importance of urease in the pathogenesis of cryptococcosis. We cloned and sequenced the genomic locus containing the single-copy C. neoformans urease gene (URE1) and used this to disrupt the native URE1 in the serotype A strain H99. The ure1 mutant strains were found to have in vitro growth characteristics, phenoloxidase activity, and capsule size similar to those of the wild type. Comparison of a ure1 mutant with H99 after intracisternal inoculation into corticosteroid-treated rabbits revealed no significant differences in colony counts recovered from the cerebrospinal fluid. However, when these two strains were compared in both the murine intravenous and inhalational infection models, there were significant differences in survival. Mice infected with a ure1 strain lived longer than mice infected with H99 in both models. The ure1 strain was restored to urease positivity by complementation with URE1, and two resulting transformants were significantly more pathogenic than the ure1 strain. Our results suggest that urease activity is involved in the pathogenesis of cryptococcosis but that the importance may be species and/or infection site specific.
Figures

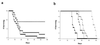
Similar articles
-
Identification of a novel gene, URE2, that functionally complements a urease-negative clinical strain of Cryptococcus neoformans.Microbiology (Reading). 2006 Dec;152(Pt 12):3723-3731. doi: 10.1099/mic.0.2006/000133-0. Microbiology (Reading). 2006. PMID: 17159224
-
Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion.Am J Pathol. 2004 May;164(5):1761-71. doi: 10.1016/S0002-9440(10)63734-0. Am J Pathol. 2004. PMID: 15111322 Free PMC article.
-
Factors required for activation of urease as a virulence determinant in Cryptococcus neoformans.mBio. 2013 May 7;4(3):e00220-13. doi: 10.1128/mBio.00220-13. mBio. 2013. PMID: 23653445 Free PMC article.
-
Cryptococcus gattii urease as a virulence factor and the relevance of enzymatic activity in cryptococcosis pathogenesis.FEBS J. 2015 Apr;282(8):1406-18. doi: 10.1111/febs.13229. Epub 2015 Mar 2. FEBS J. 2015. PMID: 25675897
-
The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans.Microbes Infect. 2003 Jun;5(7):667-75. doi: 10.1016/s1286-4579(03)00092-3. Microbes Infect. 2003. PMID: 12787743 Review.
Cited by
-
Pleiotropic effects of deubiquitinating enzyme Ubp5 on growth and pathogenesis of Cryptococcus neoformans.PLoS One. 2012;7(6):e38326. doi: 10.1371/journal.pone.0038326. Epub 2012 Jun 14. PLoS One. 2012. PMID: 22719877 Free PMC article.
-
Potential Roles of Fungal Extracellular Vesicles during Infection.mSphere. 2016 Jun 29;1(4):e00099-16. doi: 10.1128/mSphere.00099-16. eCollection 2016 Jul-Aug. mSphere. 2016. PMID: 27390779 Free PMC article. Review.
-
The Transcription Factor Pdr802 Regulates Titan Cell Formation and Pathogenicity of Cryptococcus neoformans.mBio. 2021 Mar 9;12(2):e03457-20. doi: 10.1128/mBio.03457-20. mBio. 2021. PMID: 33688010 Free PMC article.
-
Opportunistic yeast pathogens: reservoirs, virulence mechanisms, and therapeutic strategies.Cell Mol Life Sci. 2015 Jun;72(12):2261-87. doi: 10.1007/s00018-015-1860-z. Epub 2015 Feb 21. Cell Mol Life Sci. 2015. PMID: 25700837 Free PMC article. Review.
-
Role of homoserine transacetylase as a new target for antifungal agents.Antimicrob Agents Chemother. 2007 May;51(5):1731-6. doi: 10.1128/AAC.01400-06. Epub 2007 Mar 12. Antimicrob Agents Chemother. 2007. PMID: 17353245 Free PMC article.
References
-
- Bava A J, Negroni R, Bianchi M. Cryptococcosis produced by a urease negative strain of Cryptococcus neoformans. J Med Vet Mycol. 1993;31:87–89. - PubMed
-
- Canteros C E, Rodero L, Rivas M C, Davel G. A rapid urease test for presumptive identification of Cryptococcus neoformans. Mycopathologia. 1996;136:21–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

