A lipid transfer-like protein is necessary for lily pollen tube adhesion to an in vitro stylar matrix
- PMID: 10634914
- PMCID: PMC140221
- DOI: 10.1105/tpc.12.1.151
A lipid transfer-like protein is necessary for lily pollen tube adhesion to an in vitro stylar matrix
Abstract
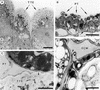
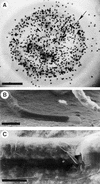
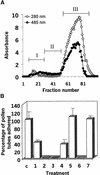
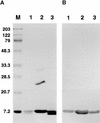
Flowering plants possess specialized extracellular matrices in the female organs of the flower that support pollen tube growth and sperm cell transfer along the transmitting tract of the gynoecium. Transport of the pollen tube cell and the sperm cells involves a cell adhesion and migration event in species such as lily that possess a transmitting tract epidermis in the stigma, style, and ovary. A bioassay for adhesion was used to isolate from the lily stigma/stylar exudate the components that are responsible for in vivo pollen tube adhesion. At least two stylar components are necessary for adhesion: a large molecule and a small (9 kD) protein. In combination, the two molecules induced adhesion of pollen tubes to an artificial stylar matrix in vitro. The 9-kD protein was purified, and its corresponding cDNA was cloned. This molecule shares some similarity with plant lipid transfer proteins. Immunolocalization data support its role in facilitating adhesion of pollen tubes to the stylar transmitting tract epidermis.
Figures




Similar articles
-
A lily stylar pectin is necessary for pollen tube adhesion to an in vitro stylar matrix.Plant Cell. 2000 Sep;12(9):1737-50. doi: 10.1105/tpc.12.9.1737. Plant Cell. 2000. PMID: 11006344 Free PMC article.
-
Expression studies of SCA in lily and confirmation of its role in pollen tube adhesion.Plant Mol Biol. 2003 Jan;51(2):183-9. doi: 10.1023/a:1021139502947. Plant Mol Biol. 2003. PMID: 12602877
-
Adhesion and cell movement during pollination: cherchez la femme.Trends Plant Sci. 2000 Sep;5(9):368-73. doi: 10.1016/s1360-1385(00)01744-1. Trends Plant Sci. 2000. PMID: 10973091 Review.
-
Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism.Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):16125-30. doi: 10.1073/pnas.2533800100. Epub 2003 Dec 11. Proc Natl Acad Sci U S A. 2003. PMID: 14671326 Free PMC article.
-
Pollen tube growth and guidance: roles of small, secreted proteins.Ann Bot. 2011 Sep;108(4):627-36. doi: 10.1093/aob/mcr015. Epub 2011 Feb 8. Ann Bot. 2011. PMID: 21307038 Free PMC article. Review.
Cited by
-
Aluminum-induced gene expression and protein localization of a cell wall-associated receptor kinase in Arabidopsis.Plant Physiol. 2003 Aug;132(4):2256-66. doi: 10.1104/pp.103.022129. Plant Physiol. 2003. PMID: 12913180 Free PMC article.
-
The Influence of Biomolecule Composition on Colloidal Beer Structure.Biomolecules. 2021 Dec 24;12(1):24. doi: 10.3390/biom12010024. Biomolecules. 2021. PMID: 35053172 Free PMC article. Review.
-
The plant stigma exudate: a biochemically active extracellular environment for pollen germination?Plant Signal Behav. 2014;9(4):e28274. doi: 10.4161/psb.28274. Epub 2014 Jan 1. Plant Signal Behav. 2014. PMID: 24589550 Free PMC article.
-
Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells.Nature. 2009 Mar 19;458(7236):357-61. doi: 10.1038/nature07882. Nature. 2009. PMID: 19295610
-
EST-based in silico identification and in vitro test of antimicrobial peptides in Brassica napus.BMC Genomics. 2015 Sep 2;16(1):653. doi: 10.1186/s12864-015-1849-x. BMC Genomics. 2015. PMID: 26330304 Free PMC article.
References
-
- Baker, B., Zambryski, P.L., Staskawicz, B., and Dinesh-Kumar, S.P. (1997). Signaling in plant–microbe interactions. Science 276 726–733. - PubMed
-
- Becraft, P.W., Stinard, P.S., and McCarty, D.R. (1996). CRINKLY4: A TNFR-like receptor kinase involved in maize epidermal differentiation. Science 273 1406–1409. - PubMed
-
- Blumenkrantz, N., and Asboe-Hansen, G. (1973). New method for quantitative determination of uronic acids. Anal. Biochem. 54 484–489. - PubMed
-
- Broekaert, W.F., Cammue, B., De Bolle, M., Thevissen, K., and De Samblanx, G. (1997). Antimicrobial peptides from plants. Crit. Rev. Plant Sci. 16 297–323.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

