Multiple domains in caveolin-1 control its intracellular traffic
- PMID: 10629215
- PMCID: PMC2156207
- DOI: 10.1083/jcb.148.1.17
Multiple domains in caveolin-1 control its intracellular traffic
Abstract
Caveolin-1 is an integral membrane protein of caveolae that is thought to play an important role in both the traffic of cholesterol to caveolae and modulating the activity of multiple signaling molecules at this site. The molecule is synthesized in the endoplasmic reticulum, transported to the cell surface, and undergoes a poorly understood recycling itinerary. We have used mutagenesis to determine the parts of the molecule that control traffic of caveolin-1 from its site of synthesis to the cell surface. We identified four regions of the molecule that appear to influence caveolin-1 traffic. A region between amino acids 66 and 70, which is in the most conserved region of the molecule, is necessary for exit from the endoplasmic reticulum. The region between amino acids 71 and 80 controls incorporation of caveolin-1 oligomers into detergent-resistant regions of the Golgi apparatus. Amino acids 91-100 and 134-154 both control oligomerization and exit from the Golgi apparatus. Removal of other portions of the molecule has no effect on targeting of newly synthesized caveolin-1 to caveolae. The results suggest that movement of caveolin-1 among various endomembrane compartments is controlled at multiple steps.
Figures

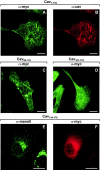

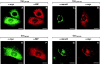
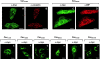


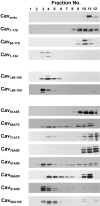
Similar articles
-
Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation.J Cell Biol. 1994 Dec;127(5):1185-97. doi: 10.1083/jcb.127.5.1185. J Cell Biol. 1994. PMID: 7962084 Free PMC article.
-
Palmitoylation of caveolin-1 is required for cholesterol binding, chaperone complex formation, and rapid transport of cholesterol to caveolae.J Biol Chem. 2000 Aug 18;275(33):25595-9. doi: 10.1074/jbc.M003401200. J Biol Chem. 2000. Retraction in: J Biol Chem. 2013 Mar 1;288(9):6585. doi: 10.1074/jbc.A113.003401 PMID: 10833523 Retracted.
-
Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities.J Biol Chem. 1997 Nov 28;272(48):30429-38. doi: 10.1074/jbc.272.48.30429. J Biol Chem. 1997. PMID: 9374534
-
Caveolins and cellular cholesterol balance.Traffic. 2000 Mar;1(3):212-7. doi: 10.1034/j.1600-0854.2000.010303.x. Traffic. 2000. PMID: 11208104 Review.
-
Intracellular cholesterol transport.J Lipid Res. 1997 Aug;38(8):1503-21. J Lipid Res. 1997. PMID: 9300773 Review.
Cited by
-
Probing the caveolin-1 P132L mutant: critical insights into its oligomeric behavior and structure.Biochemistry. 2012 May 8;51(18):3911-8. doi: 10.1021/bi3001853. Epub 2012 Apr 25. Biochemistry. 2012. PMID: 22506673 Free PMC article.
-
A disease-associated frameshift mutation in caveolin-1 disrupts caveolae formation and function through introduction of a de novo ER retention signal.Mol Biol Cell. 2017 Nov 1;28(22):3095-3111. doi: 10.1091/mbc.E17-06-0421. Epub 2017 Sep 13. Mol Biol Cell. 2017. PMID: 28904206 Free PMC article.
-
Structure and assembly of CAV1 8S complexes revealed by single particle electron microscopy.Sci Adv. 2020 Dec 2;6(49):eabc6185. doi: 10.1126/sciadv.abc6185. Print 2020 Dec. Sci Adv. 2020. PMID: 33268374 Free PMC article.
-
Tyrosine-phosphorylated caveolin-1 (Tyr-14) increases sensitivity to paclitaxel by inhibiting BCL2 and BCLxL proteins via c-Jun N-terminal kinase (JNK).J Biol Chem. 2012 May 18;287(21):17682-17692. doi: 10.1074/jbc.M111.304022. Epub 2012 Mar 20. J Biol Chem. 2012. PMID: 22433870 Free PMC article.
-
Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization.Nat Cell Biol. 2005 Sep;7(9):901-8. doi: 10.1038/ncb1293. Epub 2005 Aug 21. Nat Cell Biol. 2005. PMID: 16113676 Free PMC article.
References
-
- Anderson R.G.W. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. - PubMed
-
- Brewer C.B. Cytomegalovirus plasmid vectors for permanent lines of polarized epithelial cells. Methods Cell Biol. 1994;43:233–245. - PubMed
-
- Brown D.A., Rose J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
-
- Das K., Lewis R.Y., Scherer P.E., Lisanti M.P. The membrane-spanning domains of caveolins-1 and -2 mediate the formation of caveolin hetero-oligomers. Implications for the assembly of caveolae membranes in vivo. J. Biol. Chem. 1999;274:18721–18728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

