Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase RhoA
- PMID: 10588664
- PMCID: PMC25764
- DOI: 10.1091/mbc.10.12.4369
Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase RhoA
Abstract
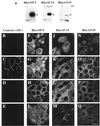
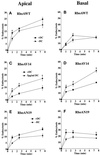
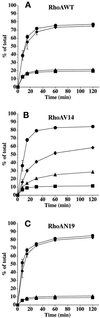
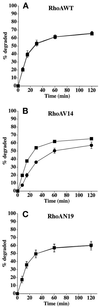
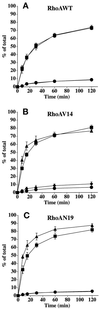
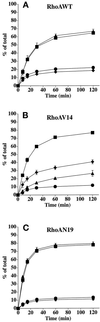
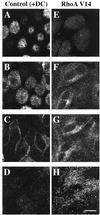
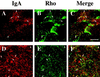
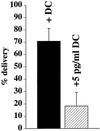
Efficient postendocytic membrane traffic in polarized epithelial cells is thought to be regulated in part by the actin cytoskeleton. RhoA modulates assemblies of actin in the cell, and it has been shown to regulate pinocytosis and phagocytosis; however, its effects on postendocytic traffic are largely unexplored. To this end, we expressed wild-type RhoA (RhoAWT), dominant active RhoA (RhoAV14), and dominant inactive RhoA (RhoAN19) in Madin-Darby canine kidney (MDCK) cells expressing the polymeric immunoglobulin receptor. RhoAV14 expression stimulated the rate of apical and basolateral endocytosis, whereas RhoAN19 expression decreased the rate from both membrane domains. Polarized basolateral recycling of transferrin was disrupted in RhoAV14-expressing cells as a result of increased ligand release at the apical pole of the cell. Degradation of basolaterally internalized epidermal growth factor was slowed in RhoAV14-expressing cells. Although apical recycling of immunoglobulin A (IgA) was largely unaffected in cells expressing RhoAV14, transcytosis of basolaterally internalized IgA was severely impaired. Morphological and biochemical analyses demonstrated that a large proportion of IgA internalized from the basolateral pole of RhoAV14-expressing cells remained within basolateral early endosomes and was slow to exit these compartments. RhoAN19 and RhoAWT expression had little effect on these postendocytic pathways. These results indicate that in polarized MDCK cells activated RhoA may modulate endocytosis from both membrane domains and postendocytic traffic at the basolateral pole of the cell.
Figures
Similar articles
-
Selective alterations in biosynthetic and endocytic protein traffic in Madin-Darby canine kidney epithelial cells expressing mutants of the small GTPase Rac1.Mol Biol Cell. 2000 Jan;11(1):287-304. doi: 10.1091/mbc.11.1.287. Mol Biol Cell. 2000. PMID: 10637309 Free PMC article.
-
Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin-Darby canine kidney cells.Mol Biol Cell. 2001 Aug;12(8):2257-74. doi: 10.1091/mbc.12.8.2257. Mol Biol Cell. 2001. PMID: 11514615 Free PMC article.
-
RhoB-dependent modulation of postendocytic traffic in polarized Madin-Darby canine kidney cells.Traffic. 2007 Jul;8(7):932-49. doi: 10.1111/j.1600-0854.2007.00575.x. Epub 2007 Jun 4. Traffic. 2007. PMID: 17547697
-
Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton.Traffic. 2001 Mar;2(3):149-59. doi: 10.1034/j.1600-0854.2001.020301.x. Traffic. 2001. PMID: 11260520 Review.
-
Regulation of endocytic traffic by rho family GTPases.Trends Cell Biol. 2000 Mar;10(3):85-8. doi: 10.1016/s0962-8924(99)01710-9. Trends Cell Biol. 2000. PMID: 10675900 Review.
Cited by
-
Cdc42 and the phosphatidylinositol 3-kinase-Akt pathway are essential for PspC-mediated internalization of pneumococci by respiratory epithelial cells.J Biol Chem. 2009 Jul 17;284(29):19427-36. doi: 10.1074/jbc.M109.003442. Epub 2009 May 27. J Biol Chem. 2009. PMID: 19473971 Free PMC article.
-
Epithelial cell polarity alters Rho-GTPase responses to Pseudomonas aeruginosa.Mol Biol Cell. 2004 Feb;15(2):411-9. doi: 10.1091/mbc.e03-08-0559. Epub 2003 Oct 31. Mol Biol Cell. 2004. PMID: 14595106 Free PMC article.
-
Macrophage/monocyte-specific deletion of Ras homolog gene family member A (RhoA) downregulates fractalkine receptor and inhibits chronic rejection of mouse cardiac allografts.J Heart Lung Transplant. 2017 Mar;36(3):340-354. doi: 10.1016/j.healun.2016.08.011. Epub 2016 Aug 20. J Heart Lung Transplant. 2017. PMID: 27692539 Free PMC article.
-
Dynamin-Independent Mechanisms of Endocytosis and Receptor Trafficking.Cells. 2022 Aug 17;11(16):2557. doi: 10.3390/cells11162557. Cells. 2022. PMID: 36010634 Free PMC article. Review.
-
Rho GTPases, phosphoinositides, and actin: a tripartite framework for efficient vesicular trafficking.Small GTPases. 2014;5:e29469. doi: 10.4161/sgtp.29469. Epub 2014 Jun 10. Small GTPases. 2014. PMID: 24914539 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

