The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes
- PMID: 10575032
- PMCID: PMC6782418
- DOI: 10.1523/JNEUROSCI.19-23-10348.1999
The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes
Abstract
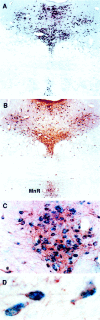
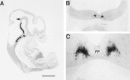
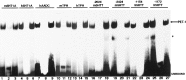
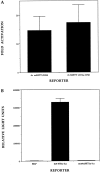
Serotonin (5-HT) plays a crucial neuromodulatory role in numerous physiological and behavioral functions, and dysfunction of the serotonergic system has been implicated in several psychiatric disorders. Despite the widespread importance of the central serotonergic neurotransmitter system, little is known about the molecular mechanisms controlling the development of 5-HT neurons. We previously identified an ETS domain transcription factor, Pet-1, that is expressed in a small number of tissues, including the brain. Here, we show that expression of Pet-1 RNA in the brain is restricted to, and marks, the entire rostrocaudal extent of rat serotonergic hindbrain raphe nuclei. Remarkably, Pet-1 RNA colocalizes with tryptophan hydroxylase-positive neurons in raphe nuclei but not with their nonserotonergic neuron or non-neuronal neighbors. Pet-1 RNA is limited to two domains in the developing hindbrain, which precedes the appearance of 5-HT in each domain by approximately a half day. Conserved Pet-1 binding sites are present in or near the promoter regions of the human and mouse 5-HT1a receptor, serotonin transporter, tryptophan hydroxylase, and aromatic L-amino acid decarboxylase genes whose expression is characteristic of the serotonergic neuron phenotype. These sites are capable of supporting transcriptional activation through interactions with the Pet-1 ETS domain and can function as enhancers. Together, our findings establish Pet-1 as an early and precise marker of 5-HT neurons and suggest that it functions specifically in the differentiation and maintenance of these neurons.
Figures


Similar articles
-
Serotonergic transcription of human FEV reveals direct GATA factor interactions and fate of Pet-1-deficient serotonin neuron precursors.J Neurosci. 2008 Nov 26;28(48):12748-58. doi: 10.1523/JNEUROSCI.4349-08.2008. J Neurosci. 2008. PMID: 19036967 Free PMC article.
-
Electrophysiological and histochemical properties of postnatal rat serotonergic neurons in dissociated cell culture.Neuroscience. 1994 Dec;63(3):775-87. doi: 10.1016/0306-4522(94)90522-3. Neuroscience. 1994. PMID: 7898677
-
Sim1 is a novel regulator in the differentiation of mouse dorsal raphe serotonergic neurons.PLoS One. 2011 Apr 26;6(4):e19239. doi: 10.1371/journal.pone.0019239. PLoS One. 2011. PMID: 21541283 Free PMC article.
-
Specification and differentiation of serotonergic neurons.Stem Cell Rev. 2006;2(1):5-10. doi: 10.1007/s12015-006-0002-2. Stem Cell Rev. 2006. PMID: 17142880 Review.
-
Developmental changes in the brain-stem serotonergic nuclei of teleost fish and neural plasticity.Cell Mol Neurobiol. 1994 Aug;14(4):381-93. doi: 10.1007/BF02088718. Cell Mol Neurobiol. 1994. PMID: 7788645 Review.
Cited by
-
Maintaining differentiated cellular identity.Nat Rev Genet. 2012 May 18;13(6):429-39. doi: 10.1038/nrg3209. Nat Rev Genet. 2012. PMID: 22596319 Review.
-
Risk of prenatal depression and stress treatment: alteration on serotonin system of offspring through exposure to Fluoxetine.Sci Rep. 2016 Oct 5;6:33822. doi: 10.1038/srep33822. Sci Rep. 2016. PMID: 27703173 Free PMC article.
-
Spatiotemporal Role of Transforming Growth Factor Beta 2 in Developing and Mature Mouse Hindbrain Serotonergic Neurons.Front Cell Neurosci. 2019 Sep 20;13:427. doi: 10.3389/fncel.2019.00427. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31619968 Free PMC article.
-
Patch-to-Seq and Transcriptomic Analyses Yield Molecular Markers of Functionally Distinct Brainstem Serotonin Neurons.Front Synaptic Neurosci. 2022 Jun 30;14:910820. doi: 10.3389/fnsyn.2022.910820. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35844900 Free PMC article.
-
Developmental Disruption of Erbb4 in Pet1+ Neurons Impairs Serotonergic Sub-System Connectivity and Memory Formation.Front Cell Dev Biol. 2021 Dec 10;9:770458. doi: 10.3389/fcell.2021.770458. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34957103 Free PMC article.
References
-
- Aitken AR, Tork I. Early development of serotonin-containing neurons and pathways as seen in wholemount preparations of the fetal rat brain. J Comp Neurol. 1988;274:32–47. - PubMed
-
- Bassuk AG, Leiden JM. The role of Ets transcription factors in the development and function of the mammalian immune system. Adv Immunol. 1997;64:65–104. - PubMed
-
- Boularand S, Darmon MC, Ravassaed P, Mallet J. Characterization of the human tryptophan hydroxylase gene promoter. J Biol Chem. 1995;270:3757–3764. - PubMed
-
- Briscoe J, Sussel L, Serup P, Hartigan-O'Connor, Jessell TM, Rubenstein JLR, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded sonic hedgehog signalling. Nature. 1999;398:622–627. - PubMed
-
- Christie GA. Developmental stages in somite and post-somite rat embryos, based on external appearance, and including some features of the macroscopic development of the oral cavity. J Morphol. 1962;114:263–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
