Improving proteolytic cleavage at the 3A/3B site of the hepatitis A virus polyprotein impairs processing and particle formation, and the impairment can be complemented in trans by 3AB and 3ABC
- PMID: 10559299
- PMCID: PMC113036
- DOI: 10.1128/JVI.73.12.9867-9878.1999
Improving proteolytic cleavage at the 3A/3B site of the hepatitis A virus polyprotein impairs processing and particle formation, and the impairment can be complemented in trans by 3AB and 3ABC
Abstract
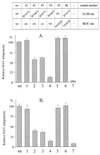
The orchestrated liberation of viral proteins by 3C(pro)-mediated proteolysis is pivotal for gene expression by picornaviruses. Proteolytic processing is regulated either by the amino acid sequence at the cleavage site of the substrate or by cofactors covalently or noncovalently linked to the viral proteinase. To determine the role of the amino acid sequence at cleavage sites 3A/3B and 3B/3C that are essential for the liberation of 3C(pro) from its precursors and to assess the function of the stable processing intermediates 3AB and 3ABC, we studied the effect of cleavage site mutations on hepatitis A virus (HAV) polyprotein processing, particle formation, and replication. Using the recombinant vaccinia virus system, we showed that the normally retarded cleavage at the 3A/3B junction can be improved by altering the amino acid sequence at the scissile bond such that it matches the preferred HAV 3C cleavage sites. In contrast to the processing products of the wild-type polyprotein, 3ABC was no longer detectable in the mutant. VP0 and VP3 were generated less efficiently, implying that processing of the structural protein precursor P1-2A depends on the presence of stable 3ABC and/or 3AB. In addition, cleavage of 2BC was impaired in 3AB/3ABC-deficient mutants. Formation of HAV particles was not affected in mutants with blocked 3A/3B and/or 3B/3C cleavage sites. However, 3ABC-deficient mutants produced small numbers of HAV particles, which could be augmented by coexpressing 3AB or 3ABC. The hydrophobic domain of 3A that has been proposed to mediate membrane anchorage of the replication complex was crucial for restoration of defective particle formation. In vitro transcripts of the various cleavage site mutants were unable to initiate an infectious cycle, and no progeny viruses were obtained even after blind passages. Taken together, the data suggest that accumulation of uncleaved HAV 3AB and/or 3ABC is pivotal for both viral replication and efficient particle formation.
Figures



Similar articles
-
Proteinase 3C of hepatitis A virus (HAV) cleaves the HAV polyprotein P2-P3 at all sites including VP1/2A and 2A/2B.Virology. 1994 Jan;198(1):275-81. doi: 10.1006/viro.1994.1030. Virology. 1994. PMID: 8259663
-
Interaction of hepatitis A virus (HAV) precursor proteins 3AB and 3ABC with the 5' and 3' termini of the HAV RNA.Virus Res. 1997 Oct;51(2):151-7. doi: 10.1016/s0168-1702(97)00089-0. Virus Res. 1997. PMID: 9498613
-
Processing of proteinase precursors and their effect on hepatitis A virus particle formation.J Virol. 1998 Oct;72(10):8013-20. doi: 10.1128/JVI.72.10.8013-8020.1998. J Virol. 1998. PMID: 9733840 Free PMC article.
-
Hepatitis A virus proteins.Intervirology. 1999;42(2-3):63-8. doi: 10.1159/000024967. Intervirology. 1999. PMID: 10516462 Review.
-
Picornavirus 3C Proteins Intervene in Host Cell Processes through Proteolysis and Interactions with RNA.Viruses. 2023 Dec 12;15(12):2413. doi: 10.3390/v15122413. Viruses. 2023. PMID: 38140654 Free PMC article. Review.
Cited by
-
Hepatitis A virus proteinase 3C binding to viral RNA: correlation with substrate binding and enzyme dimerization.Biochem J. 2005 Jan 15;385(Pt 2):363-70. doi: 10.1042/BJ20041153. Biochem J. 2005. PMID: 15361063 Free PMC article.
-
Poly(A) binding protein, C-terminally truncated by the hepatitis A virus proteinase 3C, inhibits viral translation.Nucleic Acids Res. 2007;35(17):5975-84. doi: 10.1093/nar/gkm645. Epub 2007 Aug 28. Nucleic Acids Res. 2007. PMID: 17726047 Free PMC article.
-
Increasing rate of cleavage at boundary between non-structural proteins 4B and 5A inhibits replication of hepatitis C virus.J Biol Chem. 2012 Jan 2;287(1):568-580. doi: 10.1074/jbc.M111.311407. Epub 2011 Nov 14. J Biol Chem. 2012. PMID: 22084249 Free PMC article.
-
Amino acid changes in proteins 2B and 3A mediate rhinovirus type 39 growth in mouse cells.J Virol. 2005 May;79(9):5363-73. doi: 10.1128/JVI.79.9.5363-5373.2005. J Virol. 2005. PMID: 15827151 Free PMC article.
-
Tomato ringspot virus proteins containing the nucleoside triphosphate binding domain are transmembrane proteins that associate with the endoplasmic reticulum and cofractionate with replication complexes.J Virol. 2003 Jan;77(1):523-34. doi: 10.1128/jvi.77.1.523-534.2003. J Virol. 2003. PMID: 12477857 Free PMC article.
References
-
- Beneduce, F., and G. Morace. Personal communication.
-
- Beneduce F, Ciervo A, Morace G. Site-directed mutagenesis of hepatitis A virus protein 3A: effects on membrane interaction. Biochim Biophys Acta. 1997;1326:157–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

