Replication of Mus dunni endogenous retrovirus depends on promoter activation followed by enhancer multimerization
- PMID: 10559291
- PMCID: PMC113028
- DOI: 10.1128/JVI.73.12.9803-9809.1999
Replication of Mus dunni endogenous retrovirus depends on promoter activation followed by enhancer multimerization
Abstract
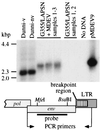
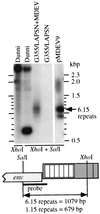
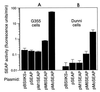
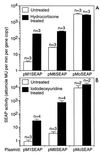
Mus dunni endogenous virus (MDEV) is an apparently intact retrovirus that normally lies transcriptionally silent in cultured M. dunni cells, but the provirus can be activated by treatment of the cells with hydrocortisone or 5-iodo-2'-deoxyuridine. Sequence analysis of a molecular clone of the replicating virus revealed a simple retrovirus with a chimeric VL30/GALV-like structure. Interestingly, in the region of the long terminal repeat (LTR) that typically contains the retroviral transcription enhancers, we found over six 80-bp repeats with only a single mismatch, indicating that acquisition of the repeats was a recent event. Here we provide evidence for the following model of MDEV activation and replication. The MDEV provirus in M. dunni cells has a chimeric structure similar to that of the molecular clone but has only 1.15 copies of the 80-bp repeat sequence found in the molecular clone. Activating chemicals directly stimulate transcription from the LTR, allowing a low level of virus replication. Copying errors made during reverse transcription allow multimerization of the 80-bp enhancer region, resulting in viruses with higher transcriptional rates and improved fitness, but increased enhancer copy number is likely balanced by the natural instability of retroviral repeats and constraints imposed by virion packaging limits. The resultant population of replicating MDEV is widely heterogeneous, having from 2.15 to 13.15 enhancer repeats in the LTR. These results reveal a novel mechanism for regulation of transcription and replication of an endogenous retrovirus, in terms of both activation of the virus by the steroid hydrocortisone and the large number and variation in enhancer repeats observed.
Figures

Similar articles
-
Sequence analysis of Mus dunni endogenous virus reveals a hybrid VL30/gibbon ape leukemia virus-like structure and a distinct envelope.J Virol. 1998 Sep;72(9):7459-66. doi: 10.1128/JVI.72.9.7459-7466.1998. J Virol. 1998. PMID: 9696842 Free PMC article.
-
Characterization of molecular clones of porcine endogenous retrovirus-A containing different numbers of U3 repeat boxes in the long terminal repeat region.J Virol Methods. 2012 Apr;181(1):103-8. doi: 10.1016/j.jviromet.2012.01.023. Epub 2012 Feb 11. J Virol Methods. 2012. PMID: 22343070
-
Molecular cloning of Mus dunni endogenous virus: an unusual retrovirus in a new murine viral interference group with a wide host range.J Virol. 1997 Jun;71(6):4663-70. doi: 10.1128/JVI.71.6.4663-4670.1997. J Virol. 1997. PMID: 9151860 Free PMC article.
-
Utility of next-generation RNA-sequencing in identifying chimeric transcription involving human endogenous retroviruses.APMIS. 2016 Jan-Feb;124(1-2):127-39. doi: 10.1111/apm.12477. APMIS. 2016. PMID: 26818267 Review.
-
Expression and regulation of human endogenous retrovirus W elements.APMIS. 2016 Jan-Feb;124(1-2):52-66. doi: 10.1111/apm.12478. APMIS. 2016. PMID: 26818262 Review.
Cited by
-
Discovery of a novel murine type C retrovirus by data mining.J Virol. 2001 Mar;75(6):3053-7. doi: 10.1128/JVI.75.6.3053-3057.2001. J Virol. 2001. PMID: 11222735 Free PMC article.
-
The GLN family of murine endogenous retroviruses contains an element competent for infectious viral particle formation.J Virol. 2008 May;82(9):4413-9. doi: 10.1128/JVI.02141-07. Epub 2008 Feb 20. J Virol. 2008. PMID: 18287236 Free PMC article.
-
High-efficiency promoter-dependent transduction by adeno-associated virus type 6 vectors in mouse lung.Hum Gene Ther. 2007 Apr;18(4):344-54. doi: 10.1089/hum.2006.182. Hum Gene Ther. 2007. PMID: 17430088 Free PMC article.
-
The number of a U3 repeat box acting as an enhancer in long terminal repeats of polytropic replication-competent porcine endogenous retroviruses dynamically fluctuates during serial virus passages in human cells.J Virol. 2001 Aug;75(15):6933-40. doi: 10.1128/JVI.75.15.6933-6940.2001. J Virol. 2001. PMID: 11435573 Free PMC article.
-
Genome structure and thymic expression of an endogenous retrovirus in zebrafish.J Virol. 2004 Jan;78(2):899-911. doi: 10.1128/jvi.78.2.899-911.2004. J Virol. 2004. PMID: 14694121 Free PMC article.
References
-
- Berger J, Hauber J, Hauber R, Geiger R, Cullen B R. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988;66:1–10. - PubMed
-
- Biegalke B J, Geballe A P. Translational inhibition by cytomegalovirus transcript leaders. Virology. 1990;177:657–667. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

