Isolation of a Chinese hamster ovary cell mutant defective in intramitochondrial transport of phosphatidylserine
- PMID: 10535934
- PMCID: PMC22930
- DOI: 10.1073/pnas.96.22.12400
Isolation of a Chinese hamster ovary cell mutant defective in intramitochondrial transport of phosphatidylserine
Abstract
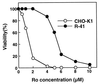
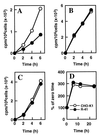
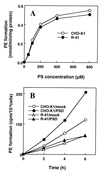
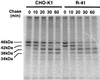
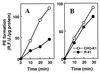
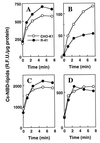
A CHO-K1 cell mutant with a specific decrease in cellular phosphatidylethanolamine (PE) level was isolated as a variant resistant to Ro09-0198, a PE-directed antibiotic peptide. The mutant was defective in the phosphatidylserine (PS) decarboxylation pathway for PE formation, in which PS produced in the endoplasmic reticulum is transported to mitochondria and then decarboxylated by an inner mitochondrial membrane enzyme, PS decarboxylase. Neither PS formation nor PS decarboxylase activity was reduced in the mutant, implying that the mutant is defective in some step of PS transport. The transport processes of phospholipids between the outer and inner mitochondrial membrane were analyzed by use of isolated mitochondria and two fluorescence-labeled phospholipid analogs, 1-palmitoyl-2-[N-[6(7-nitrobenz-2-oxa-1, 3-diazol-4-yl)amino]caproyl]-PS (C6-NBD-PS) and C6-NBD-phosphatidylcholine (C6-NBD-PC). On incubation with the CHO-K1 mitochondria, C6-NBD-PS was readily decarboxylated to C6-NBD-PE, suggesting that the PS analog was partitioned into the outer leaflet of mitochondria and then translocated to the inner mitochondrial membrane. The rate of decarboxylation of C6-NBD-PS in the mutant mitochondria was reduced to approximately 40% of that in the CHO-K1 mitochondria. The quantity of phospholipid analogs translocated from the outer leaflet of mitochondria into inner mitochondrial membranes was further examined by selective extraction of the analogs from the outer leaflet of mitochondria. In the mutant mitochondria, the translocation of C6-NBD-PS was significantly reduced, whereas the translocation of C6-NBD-PC was not affected. These results indicate that the mutant is defective in PS transport between the outer and inner mitochondrial membrane and provide genetic evidence for the existence of a specific mechanism for intramitochondrial transport of PS.
Figures
Similar articles
-
A Chinese hamster ovary cell mutant defective in the non-endocytic uptake of fluorescent analogs of phosphatidylserine: isolation using a cytosol acidification protocol.J Cell Biol. 1995 Mar;128(5):793-804. doi: 10.1083/jcb.128.5.793. J Cell Biol. 1995. PMID: 7876305 Free PMC article.
-
Intramitochondrial distribution and transport of phosphatidylserine and its decarboxylation product, phosphatidylethanolamine. Application of pyrene-labeled species.Biochim Biophys Acta. 1993 Oct 10;1152(1):161-70. doi: 10.1016/0005-2736(93)90243-s. Biochim Biophys Acta. 1993. PMID: 8399295
-
Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells.Biochim Biophys Acta. 2013 Mar;1831(3):543-54. doi: 10.1016/j.bbalip.2012.08.016. Epub 2012 Aug 29. Biochim Biophys Acta. 2013. PMID: 22960354 Review.
-
Phosphatidylserine flux into mitochondria unveiled by organelle-targeted Escherichia coli phosphatidylserine synthase PssA.FEBS J. 2021 May;288(10):3285-3299. doi: 10.1111/febs.15657. Epub 2020 Dec 30. FEBS J. 2021. PMID: 33283454
-
Molecular and cell biology of phosphatidylserine and phosphatidylethanolamine metabolism.Prog Nucleic Acid Res Mol Biol. 2003;75:69-111. doi: 10.1016/s0079-6603(03)75003-x. Prog Nucleic Acid Res Mol Biol. 2003. PMID: 14604010 Review.
Cited by
-
Lipid transport between the endoplasmic reticulum and mitochondria.Cold Spring Harb Perspect Biol. 2013 Jun 1;5(6):a013235. doi: 10.1101/cshperspect.a013235. Cold Spring Harb Perspect Biol. 2013. PMID: 23732475 Free PMC article. Review.
-
Role for phospholipid flippase complex of ATP8A1 and CDC50A proteins in cell migration.J Biol Chem. 2013 Feb 15;288(7):4922-34. doi: 10.1074/jbc.M112.402701. Epub 2012 Dec 26. J Biol Chem. 2013. PMID: 23269685 Free PMC article.
-
Vesicular and nonvesicular transport of ceramide from ER to the Golgi apparatus in yeast.J Cell Biol. 2001 Dec 10;155(6):949-59. doi: 10.1083/jcb.200105033. Epub 2001 Dec 3. J Cell Biol. 2001. PMID: 11733544 Free PMC article.
-
Overexpression of ERp29 in the thyrocytes of FRTL-5 cells.Mol Biol Rep. 2005 Mar;32(1):7-13. doi: 10.1007/s11033-004-3069-3. Mol Biol Rep. 2005. PMID: 15865205
-
Making the final cut - mechanisms mediating the abscission step of cytokinesis.ScientificWorldJournal. 2010 Jul 19;10:1424-34. doi: 10.1100/tsw.2010.129. ScientificWorldJournal. 2010. PMID: 20661535 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

