PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1
- PMID: 10525530
- PMCID: PMC2174231
- DOI: 10.1083/jcb.147.2.221
PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1
Abstract
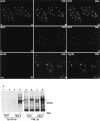
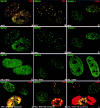
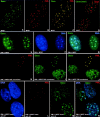
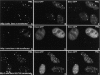
Nuclear domain 10 (ND10), also referred to as nuclear bodies, are discrete interchromosomal accumulations of several proteins including promyelocytic leukemia protein (PML) and Sp100. In this study, we investigated the mechanism of ND10 assembly by identifying proteins that are essential for this process using cells lines that lack individual ND10-associated proteins. We identified the adapter protein Daxx and BML, the RecQ helicase missing in Bloom syndrome, as new ND10-associated proteins. PML, but not BLM or Sp100, was found to be responsible for the proper localization of all other ND10-associated proteins since they are dispersed in PML-/- cells. Introducing PML into this cell line by transient expression or fusion with PML-producing cells recruited ND10-associated proteins into de novo formed ND10 attesting to PMLs essential nature in ND10 formation. In the absence of PML, Daxx is highly enriched in condensed chromatin. Its recruitment to ND10 from condensed chromatin requires a small ubiquitin-related modifier (SUMO-1) modification of PML and reflects the interaction between the COOH-terminal domain of Daxx and PML. The segregation of Daxx from condensed chromatin in the absence of PML to ND10 by increased accumulation of SUMO-1-modified PML suggests the presence of a variable equilibrium between these two nuclear sites. Our findings identify the basic requirements for ND10 formation and suggest a dynamic mechanism for protein recruitment to these nuclear domains controlled by the SUMO-1 modification state of PML.
Figures

Similar articles
-
Review: properties and assembly mechanisms of ND10, PML bodies, or PODs.J Struct Biol. 2000 Apr;129(2-3):278-87. doi: 10.1006/jsbi.2000.4239. J Struct Biol. 2000. PMID: 10806078 Review.
-
The homeodomain-interacting kinase PKM (HIPK-2) modifies ND10 through both its kinase domain and a SUMO-1 interaction motif and alters the posttranslational modification of PML.Exp Cell Res. 2003 Feb 1;283(1):36-50. doi: 10.1016/s0014-4827(02)00025-3. Exp Cell Res. 2003. PMID: 12565818
-
Heat shock and Cd2+ exposure regulate PML and Daxx release from ND10 by independent mechanisms that modify the induction of heat-shock proteins 70 and 25 differently.J Cell Sci. 2003 Feb 1;116(Pt 3):513-24. doi: 10.1242/jcs.00253. J Cell Sci. 2003. PMID: 12508112
-
Role of SUMO-1-modified PML in nuclear body formation.Blood. 2000 May 1;95(9):2748-52. Blood. 2000. PMID: 10779416
-
PML NBs (ND10) and Daxx: from nuclear structure to protein function.Front Biosci. 2008 May 1;13:7132-42. doi: 10.2741/3216. Front Biosci. 2008. PMID: 18508722 Review.
Cited by
-
A comprehensive compilation of SUMO proteomics.Nat Rev Mol Cell Biol. 2016 Sep;17(9):581-95. doi: 10.1038/nrm.2016.81. Epub 2016 Jul 20. Nat Rev Mol Cell Biol. 2016. PMID: 27435506
-
Single-cell analysis of Daxx and ATRX-dependent transcriptional repression.J Cell Sci. 2012 Nov 15;125(Pt 22):5489-501. doi: 10.1242/jcs.110148. Epub 2012 Sep 12. J Cell Sci. 2012. PMID: 22976303 Free PMC article.
-
Contribution of the Major ND10 Proteins PML, hDaxx and Sp100 to the Regulation of Human Cytomegalovirus Latency and Lytic Replication in the Monocytic Cell Line THP-1.Viruses. 2015 Jun 5;7(6):2884-907. doi: 10.3390/v7062751. Viruses. 2015. PMID: 26057166 Free PMC article.
-
Nuclear domain 10-associated proteins recognize and segregate intranuclear DNA/protein complexes to negate gene expression.Virol J. 2012 Sep 28;9:222. doi: 10.1186/1743-422X-9-222. Virol J. 2012. PMID: 23021128 Free PMC article.
-
Assembly of Epstein-Barr Virus Capsid in Promyelocytic Leukemia Nuclear Bodies.J Virol. 2015 Sep;89(17):8922-31. doi: 10.1128/JVI.01114-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085145 Free PMC article.
References
-
- Ahn J.H., Brignole E.J., III, Hayward G.S. Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol. Cell Biol. 1998;18:4899–4913. - PMC - PubMed
-
- Boddy M.N., Howe K., Etkin L.D., Solomon E., Freemont P.S. PIC 1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene. 1996;13:971–982. - PubMed
-
- Chelbi-Alix M.K., de The H. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene. 1999;18:935–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

