Selective expression of the large neutral amino acid transporter at the blood-brain barrier
- PMID: 10518579
- PMCID: PMC18415
- DOI: 10.1073/pnas.96.21.12079
Selective expression of the large neutral amino acid transporter at the blood-brain barrier
Abstract
Amino acid supply in brain is regulated by the activity of the large neutral amino acid transporter (LAT) at the brain capillary endothelial cell, which forms the blood-brain barrier (BBB) in vivo. Bovine BBB poly(A)(+) RNA was isolated from 2.0 kg of fresh bovine brain and size fractionated on a sucrose density gradient, and a size-fractionated bovine BBB cDNA library in the pSPORT vector was prepared. The full-length cDNA encoding the bovine BBB LAT was isolated from this library, and the predicted amino acid sequence was 89-92% identical to the LAT1 isoform. The bovine BBB LAT1 mRNA produced a 10-fold enhancement in tryptophan transport into frog oocytes coinjected with bovine BBB LAT1 mRNA and the mRNA for 4F2hc, which encodes the heavy chain of the heterodimer. Tryptophan transport into the mRNA-injected oocytes was sodium independent and was specifically inhibited by other large neutral amino acids, and the K(m) of tryptophan transport was 31.5 +/- 5.5 microM. Northern blotting with the bovine BBB LAT1 cDNA showed that the LAT1 mRNA is 100-fold higher in isolated bovine brain capillaries compared with C6 rat glioma cells or rat brain, and the LAT1 mRNA was not detected in rat liver, heart, lung, or kidney. These studies show that the LAT1 transcript is selectively expressed at the BBB compared with other tissues, and the abundance of the LAT1 mRNA at the BBB is manyfold higher than that of transcripts such as the 4F2hc antigen, actin, or the Glut1 glucose transporter.
Figures

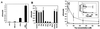

Similar articles
-
Site-directed mutagenesis of rabbit LAT1 at amino acids 219 and 234.J Neurochem. 2003 Mar;84(6):1322-31. doi: 10.1046/j.1471-4159.2003.01622.x. J Neurochem. 2003. PMID: 12614332
-
The 4F2hc/LAT1 complex transports L-DOPA across the blood-brain barrier.Brain Res. 2000 Oct 6;879(1-2):115-21. doi: 10.1016/s0006-8993(00)02758-x. Brain Res. 2000. PMID: 11011012
-
Hypoxia induces de-stabilization of the LAT1 large neutral amino acid transporter mRNA in brain capillary endothelial cells.J Neurochem. 2003 May;85(4):1037-42. doi: 10.1046/j.1471-4159.2003.01757.x. J Neurochem. 2003. PMID: 12716435
-
Neutral amino acid transport at the human blood-brain barrier.Fed Proc. 1986 Jun;45(7):2073-8. Fed Proc. 1986. PMID: 3519291 Review.
-
The role of blood-brain barrier transport of tryptophan and other neutral amino acids in the regulation of substrate-limited pathways of brain amino acid metabolism.J Neural Transm Suppl. 1979;(15):43-54. doi: 10.1007/978-3-7091-2243-3_4. J Neural Transm Suppl. 1979. PMID: 385809 Review.
Cited by
-
Transport of Amino Acids Across the Blood-Brain Barrier.Front Physiol. 2020 Sep 23;11:973. doi: 10.3389/fphys.2020.00973. eCollection 2020. Front Physiol. 2020. PMID: 33071801 Free PMC article. Review.
-
Blood-Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles.Pharmaceutics. 2022 Oct 19;14(10):2237. doi: 10.3390/pharmaceutics14102237. Pharmaceutics. 2022. PMID: 36297671 Free PMC article. Review.
-
The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy.J Clin Neurosci. 2015 Dec;22(12):1964-8. doi: 10.1016/j.jocn.2015.06.018. Epub 2015 Aug 14. J Clin Neurosci. 2015. PMID: 26279502 Free PMC article.
-
Activation of system L heterodimeric amino acid exchangers by intracellular substrates.EMBO J. 2002 Feb 15;21(4):580-9. doi: 10.1093/emboj/21.4.580. EMBO J. 2002. PMID: 11847106 Free PMC article.
-
Hemostasis and alterations of the central nervous system.Semin Thromb Hemost. 2013 Nov;39(8):856-75. doi: 10.1055/s-0033-1357490. Epub 2013 Oct 28. Semin Thromb Hemost. 2013. PMID: 24166247 Free PMC article. Review.
References
-
- Pardridge W M. Physiol Rev. 1983;63:1481–1535. - PubMed
-
- Lund-Anderson H. Physiol Rev. 1979;59:305–352. - PubMed
-
- Christensen H N. J Exp Biol. 1994;196:51–57. - PubMed
-
- Miller L P, Pardridge W M, Braun L D, Oldendorf W H. J Neurochem. 1985;45:1427–1432. - PubMed
-
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. J Biol Chem. 1998;273:23629–23632. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

