Cloning and molecular analyses of a gibberellin 20-oxidase gene expressed specifically in developing seeds of watermelon
- PMID: 10517828
- PMCID: PMC59399
- DOI: 10.1104/pp.121.2.373
Cloning and molecular analyses of a gibberellin 20-oxidase gene expressed specifically in developing seeds of watermelon
Abstract
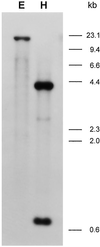
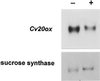
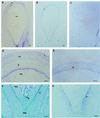
To understand the biosynthesis and functional role of gibberellins (GAs) in developing seeds, we isolated Cv20ox, a cDNA clone from watermelon (Citrullus lanatus) that shows significant amino acid homology with GA 20-oxidases. The complementary DNA clone was expressed in Escherichia coli as a fusion protein, which oxidized GA(12) at C-20 to the C(19) compound GA(9), a precursor of bioactive GAs. RNA-blot analysis showed that the Cv20ox gene was expressed specifically in developing seeds. The gene was strongly expressed in the integument tissues, and it was also expressed weakly in inner seed tissues. In parthenocarpic fruits induced by 1-(2-chloro-4-pyridyl)-3-phenylurea treatment, the expression pattern of Cv20ox did not change, indicating that the GA 20-oxidase gene is expressed primarily in the maternal cells of developing seeds. The promoter of Cv20ox was isolated and fused to the beta-glucuronidase (GUS) gene. In a transient expression system, beta-glucuronidase staining was detectable only in the integument tissues of developing watermelon seeds.
Figures



Similar articles
-
Expression studies of gibberellin oxidases in developing pumpkin seeds.Plant Physiol. 2003 Mar;131(3):1220-7. doi: 10.1104/pp.015206. Plant Physiol. 2003. PMID: 12644672 Free PMC article.
-
Cloning of gibberellin 3 beta-hydroxylase cDNA and analysis of endogenous gibberellins in the developing seeds in watermelon.Plant Cell Physiol. 2002 Feb;43(2):152-8. doi: 10.1093/pcp/pcf016. Plant Cell Physiol. 2002. PMID: 11867694
-
CvADH1, a member of short-chain alcohol dehydrogenase family, is inducible by gibberellin and sucrose in developing watermelon seeds.Plant Cell Physiol. 2003 Jan;44(1):85-92. doi: 10.1093/pcp/pcg013. Plant Cell Physiol. 2003. PMID: 12552151
-
The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: molecular cloning and functional expression.Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6640-4. doi: 10.1073/pnas.92.14.6640. Proc Natl Acad Sci U S A. 1995. PMID: 7604047 Free PMC article.
-
The gene encoding tobacco gibberellin 3beta-hydroxylase is expressed at the site of GA action during stem elongation and flower organ development.Plant J. 1999 Oct;20(1):15-24. doi: 10.1046/j.1365-313x.1999.00568.x. Plant J. 1999. PMID: 10571861
Cited by
-
Molecular cloning of GA 2-oxidase3 from spinach and its ectopic expression in Nicotiana sylvestris.Plant Physiol. 2005 May;138(1):243-54. doi: 10.1104/pp.104.056499. Epub 2005 Apr 8. Plant Physiol. 2005. PMID: 15821147 Free PMC article.
-
Immunohistochemistry of active gibberellins and gibberellin-inducible alpha-amylase in developing seeds of morning glory.Plant Physiol. 2002 Jul;129(3):1045-53. doi: 10.1104/pp.010921. Plant Physiol. 2002. PMID: 12114559 Free PMC article.
-
OsPhyA modulates rice flowering time mainly through OsGI under short days and Ghd7 under long days in the absence of phytochrome B.Plant Mol Biol. 2016 Jul;91(4-5):413-27. doi: 10.1007/s11103-016-0474-7. Epub 2016 Apr 2. Plant Mol Biol. 2016. PMID: 27039184
-
Gibberellin-to-abscisic acid balances govern development and differentiation of the nucellar projection of barley grains.J Exp Bot. 2014 Oct;65(18):5291-304. doi: 10.1093/jxb/eru289. Epub 2014 Jul 14. J Exp Bot. 2014. PMID: 25024168 Free PMC article.
-
Expression studies of gibberellin oxidases in developing pumpkin seeds.Plant Physiol. 2003 Mar;131(3):1220-7. doi: 10.1104/pp.015206. Plant Physiol. 2003. PMID: 12644672 Free PMC article.
References
-
- Ait-Ali T, Swain SM, Reid JB, Sun T, Kamiya Y. The LS locus of pea encodes the gibberellin biosynthesis enzyme ent-kaurene synthase A. Plant J. 1997;11:443–454. - PubMed
-
- Albert S, Delseny M, Devic M. BANYULS, a novel negative regulator of flavonoid biosynthesis in the Arabidopsis seed coat. Plant J. 1997;11:289–299. - PubMed
-
- Barendse GWM, Kepczynski J, Karssen CM, Koornneef M. The role of endogenous gibberellins during fruit and seed development: studies on gibberellin-deficient genotypes of Arabidopsis thaliana. Physiol Plant. 1986;67:315–319.
-
- Dejardin A, Rochat C, Maugenest S, Boutin J-P. Purification, characterization and physiological role of sucrose synthase in the pea seed coat (Pisum sativum L.) Planta. 1997;201:128–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

