Multiple, targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment
- PMID: 10500197
- PMCID: PMC18054
- DOI: 10.1073/pnas.96.20.11452
Multiple, targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment
Abstract
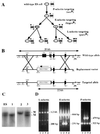
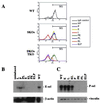
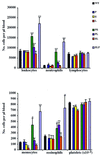
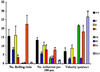
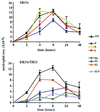
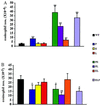
We extend our previous analyses of mice deficient in selectins by describing the generation and comparative phenotype of mice lacking one, two, or three selectins after sequential ablation of the murine genes encoding P-, E-, and L-selectins. All mice deficient in selectins are viable and fertile as homozygotes. However, mice missing both P- and E-selectins (PE(-/-)), and mice missing all three selectins (ELP(-/-)) develop mucocutaneous infections that eventually lead to death. Mice deficient in multiple selectins display varying degrees of leukocytosis, resulting in part from alterations in leukocyte rolling and recruitment. PE(-/-) mice, ELP(-/-) mice, and mice missing both P- and L-selectins (PL(-/-)) show drastic reductions in leukocyte rolling and in extravasation of neutrophils in thioglycollate-induced peritonitis. In a separate inflammatory model (ragweed-induced peritoneal eosinophilia), we demonstrate P-selectin to be both necessary and sufficient for the recruitment of eosinophils. The phenotype of mice missing both E- and L-selectins (EL(-/-)) is less severe than those seen in the other double knockouts. Comparisons among the double knockouts suggest that P-selectin normally cooperates with both E- and L-selectins. Our results indicate a preeminent role for P-selectin in regulating leukocyte behavior in mice. Data from the ELP(-/-) mice indicate, however, that all three selectins are important to leukocyte homeostasis and efficient neutrophil recruitment.
Figures
Similar articles
-
Mice lacking two or all three selectins demonstrate overlapping and distinct functions for each selectin.J Immunol. 1999 Jun 1;162(11):6755-62. J Immunol. 1999. PMID: 10352295
-
Gene-targeted mice reveal importance of L-selectin-dependent rolling for neutrophil adhesion.Am J Physiol. 1998 May;274(5):H1785-91. doi: 10.1152/ajpheart.1998.274.5.H1785. Am J Physiol. 1998. PMID: 9612391
-
Insights into selectin function from knockout mice.Thromb Haemost. 1997 Jul;78(1):60-4. Thromb Haemost. 1997. PMID: 9198128 Review.
-
Selectin-independent leukocyte rolling and adhesion in mice deficient in E-, P-, and L-selectin and ICAM-1.Am J Physiol Heart Circ Physiol. 2001 Feb;280(2):H634-41. doi: 10.1152/ajpheart.2001.280.2.H634. Am J Physiol Heart Circ Physiol. 2001. PMID: 11158961
-
New discoveries with mice mutant in endothelial and platelet selectins.Thromb Haemost. 1999 Aug;82(2):850-7. Thromb Haemost. 1999. PMID: 10605793 Review.
Cited by
-
Suppressive effects of selectin inhibitor SKK-60060 on the leucocyte infiltration during endotoxin induced uveitis.Br J Ophthalmol. 2003 Apr;87(4):476-80. doi: 10.1136/bjo.87.4.476. Br J Ophthalmol. 2003. PMID: 12642314 Free PMC article.
-
Leukocyte recruitment and the acute inflammatory response.Brain Pathol. 2000 Jan;10(1):127-35. doi: 10.1111/j.1750-3639.2000.tb00249.x. Brain Pathol. 2000. PMID: 10668902 Free PMC article. Review.
-
Increased primary tumor growth in mice null for beta3- or beta3/beta5-integrins or selectins.Proc Natl Acad Sci U S A. 2004 Jan 20;101(3):763-8. doi: 10.1073/pnas.0307289101. Epub 2004 Jan 12. Proc Natl Acad Sci U S A. 2004. PMID: 14718670 Free PMC article.
-
LPS-induced Lung Platelet Recruitment Occurs Independently from Neutrophils, PSGL-1, and P-Selectin.Am J Respir Cell Mol Biol. 2019 Aug;61(2):232-243. doi: 10.1165/rcmb.2018-0182OC. Am J Respir Cell Mol Biol. 2019. PMID: 30768917 Free PMC article.
-
Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking.J Biol Chem. 2011 Mar 4;286(9):7348-58. doi: 10.1074/jbc.M110.171819. Epub 2010 Dec 20. J Biol Chem. 2011. PMID: 21173151 Free PMC article.
References
-
- Vestweber D, Blanks J. Physiol Rev. 1999;79:181–213. - PubMed
-
- Mayadas T N, Johnson R C, Rayburn H, Hynes R O, Wagner D D. Cell. 1993;74:541–554. - PubMed
-
- Arbones M L, Ord D C, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon D J, Tedder T F. Immunity. 1994;1:247–260. - PubMed
-
- Labow M A, Norton C R, Rumberger J M, Lombard-Gillooly K M, Shuster D J, Hubbard J, Bertko R, Knaack P A, Terry R W, Harbison M L, et al. Immunity. 1994;1:709–720. - PubMed
-
- Frenette P S, Mayadas T N, Rayburn H, Hynes R O, Wagner D D. Cell. 1996;84:563–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

