Platelet-derived growth factor beta receptor regulates interstitial fluid homeostasis through phosphatidylinositol-3' kinase signaling
- PMID: 10500190
- PMCID: PMC18047
- DOI: 10.1073/pnas.96.20.11410
Platelet-derived growth factor beta receptor regulates interstitial fluid homeostasis through phosphatidylinositol-3' kinase signaling
Abstract
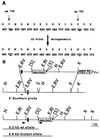
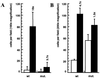
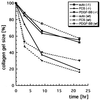
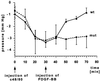
Platelet-derived growth factor (PDGF) isoforms lead to mitogenic, survival, and chemotactic responses in a variety of mesenchymal cell types during development and in the adult. We have studied the importance of phosphatidylinositol-3' kinase (PI3K) signaling in these responses by mutating the PI3K-binding sites in the PDGF-beta receptor by gene targeting in embryonic stem cells. Homozygous mutant mice developed normally; however, cells derived from the mutants were less chemotactic and had largely lost their ability to contract collagen gels in response to PDGF. Injection of a mast cell degranulating agent in mice led to a decrease in interstitial fluid pressure resulting in edema formation. In contrast to wild-type mice, mutant mice were unable to normalize the pressure after treatment with PDGF. Taken together, these observations suggest a function for PDGF signaling through PI3K in interstitial fluid homeostasis by modulating the tension between cells and extracellular matrix structures.
Figures


Similar articles
-
Mutation of a Src phosphorylation site in the PDGF beta-receptor leads to increased PDGF-stimulated chemotaxis but decreased mitogenesis.EMBO J. 1996 Oct 1;15(19):5299-313. EMBO J. 1996. PMID: 8895575 Free PMC article.
-
The kinase-inactive PDGF beta-receptor mediates activation of the MAP kinase cascade via the endogenous PDGF alpha-receptor in HepG2 cells.Oncogene. 1996 Jul 4;13(1):151-9. Oncogene. 1996. PMID: 8700541
-
Platelet-derived growth factor-mediated signaling through the Shb adaptor protein: effects on cytoskeletal organization.Exp Cell Res. 2000 Jun 15;257(2):245-54. doi: 10.1006/excr.2000.4896. Exp Cell Res. 2000. PMID: 10837138
-
Loop III region of platelet-derived growth factor (PDGF) B-chain mediates binding to PDGF receptors and heparin.Biochem J. 1998 Aug 1;333 ( Pt 3)(Pt 3):637-44. doi: 10.1042/bj3330637. Biochem J. 1998. PMID: 9677323 Free PMC article.
-
Functional analysis of aortic endothelial cells expressing mutant PDGF receptors with respect to expression of matrix metalloproteinase-3.Biochem Biophys Res Commun. 2002 Jun 7;294(2):231-7. doi: 10.1016/S0006-291X(02)00468-0. Biochem Biophys Res Commun. 2002. PMID: 12051699
Cited by
-
Blood vessels as targets in tumor therapy.Ups J Med Sci. 2012 May;117(2):178-86. doi: 10.3109/03009734.2012.660550. Epub 2012 Feb 21. Ups J Med Sci. 2012. PMID: 22348394 Free PMC article. Review.
-
[Skin changes as a result of targeted therapies in oncology patients: cutaneous side effects of targeted therapies in oncology patients].Hautarzt. 2009 May;60(5):433-40. doi: 10.1007/s00105-009-1754-9. Hautarzt. 2009. PMID: 19430753 Review. German.
-
Increased serum and musculotendinous fibrogenic proteins following persistent low-grade inflammation in a rat model of long-term upper extremity overuse.PLoS One. 2013 Aug 28;8(8):e71875. doi: 10.1371/journal.pone.0071875. eCollection 2013. PLoS One. 2013. PMID: 24015193 Free PMC article.
-
Safety and pharmacokinetics of dose-intensive imatinib mesylate plus temozolomide: phase 1 trial in adults with malignant glioma.Neuro Oncol. 2008 Jun;10(3):330-40. doi: 10.1215/15228517-2008-003. Epub 2008 Mar 21. Neuro Oncol. 2008. PMID: 18359865 Free PMC article. Clinical Trial.
-
LRP1 regulates architecture of the vascular wall by controlling PDGFRbeta-dependent phosphatidylinositol 3-kinase activation.PLoS One. 2009 Sep 9;4(9):e6922. doi: 10.1371/journal.pone.0006922. PLoS One. 2009. PMID: 19742316 Free PMC article.
References
-
- Heldin C-H, Westermark B. In: The Molecular and Cellular Biology of Wound Repair. Clark R A F, editor. New York: Plenum; 1996. pp. 249–273.
-
- Heldin C H, Östman A, Ronnstrand L. Biochim Biophys Acta. 1998;1378:F79–F113. - PubMed
-
- Rosenkranz S, Kazlauskas A. Growth Factors. 1999;16:201–216. - PubMed
-
- Kundra V, Escobedo J A, Kazlauskas A, Kim H K, Rhee S G, Williams L T, Zetter B R. Nature (London) 1994;367:474–476. - PubMed
-
- Wennström S, Siegbahn A, Yokote K, Arvidsson A-K, Heldin C-H, Mori S, Claesson-Welsh L. Oncogene. 1994;9:651–660. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

