Deletion of Ku86 causes early onset of senescence in mice
- PMID: 10485901
- PMCID: PMC17958
- DOI: 10.1073/pnas.96.19.10770
Deletion of Ku86 causes early onset of senescence in mice
Abstract
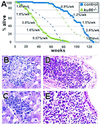
DNA double-strand breaks formed during the assembly of antigen receptors or after exposure to ionizing radiation are repaired by proteins important for nonhomologous end joining that include Ku86, Ku70, DNA-PK(CS), Xrcc4, and DNA ligase IV. Here we show that ku86-mutant mice, compared with control littermates, prematurely exhibited age-specific changes characteristic of senescence that include osteopenia, atrophic skin, hepatocellular degeneration, hepatocellular inclusions, hepatic hyperplastic foci, and age-specific mortality. Cancer and likely sepsis (indicated by reactive immune responses) partly contributed to age-specific mortality for both cohorts, and both conditions occurred earlier in ku86(-/-) mice. These data indicate that Ku86-dependent chromosomal metabolism is important for determining the onset of age-specific changes characteristic of senescence in mice.
Figures


Similar articles
-
Protein-protein and protein-DNA interaction regions within the DNA end-binding protein Ku70-Ku86.Mol Cell Biol. 1996 Sep;16(9):5186-93. doi: 10.1128/MCB.16.9.5186. Mol Cell Biol. 1996. PMID: 8756676 Free PMC article.
-
The nonhomologous DNA end joining pathway is important for chromosome stability in primary fibroblasts.Curr Biol. 1999 Dec 16-30;9(24):1501-4. doi: 10.1016/s0960-9822(00)80123-2. Curr Biol. 1999. PMID: 10607596
-
Mammalian Ku86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang.EMBO Rep. 2000 Sep;1(3):244-52. doi: 10.1093/embo-reports/kvd051. EMBO Rep. 2000. PMID: 11256607 Free PMC article.
-
The biochemistry and biological significance of nonhomologous DNA end joining: an essential repair process in multicellular eukaryotes.Genes Cells. 1999 Feb;4(2):77-85. doi: 10.1046/j.1365-2443.1999.00245.x. Genes Cells. 1999. PMID: 10320474 Review.
-
Tying loose ends: roles of Ku and DNA-dependent protein kinase in the repair of double-strand breaks.Curr Opin Genet Dev. 1997 Feb;7(1):99-104. doi: 10.1016/s0959-437x(97)80116-5. Curr Opin Genet Dev. 1997. PMID: 9024627 Review.
Cited by
-
Stem cells, senescence, neosis and self-renewal in cancer.Cancer Cell Int. 2006 Nov 8;6:25. doi: 10.1186/1475-2867-6-25. Cancer Cell Int. 2006. PMID: 17092342 Free PMC article.
-
Ku86 is essential in human somatic cells.Proc Natl Acad Sci U S A. 2002 Jan 22;99(2):832-7. doi: 10.1073/pnas.022649699. Epub 2002 Jan 15. Proc Natl Acad Sci U S A. 2002. PMID: 11792868 Free PMC article.
-
Ku complex interacts with and stimulates the Werner protein.Genes Dev. 2000 Apr 15;14(8):907-12. Genes Dev. 2000. PMID: 10783163 Free PMC article.
-
The absence of the dna-dependent protein kinase catalytic subunit in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang.Mol Cell Biol. 2001 Jun;21(11):3642-51. doi: 10.1128/MCB.21.11.3642-3651.2001. Mol Cell Biol. 2001. PMID: 11340158 Free PMC article.
-
DNA Repair Defects and DNA-PK in Neurodegeneration.Cell Dev Biol. 2012;1(2):1000e105. doi: 10.4172/2168-9296. Epub 2012 May 25. Cell Dev Biol. 2012. PMID: 28066691 Free PMC article. No abstract available.
References
-
- Martin G M. Birth Defects Orig Artic Ser. 1978;14:5–39. - PubMed
-
- Epstein C J, Martin G M, Schultz A L, Motulsky A G. Medicine. 1966;45:177–221. - PubMed
-
- Yu C-E, Oshima J, Fu Y-H, Wijsman E M, Hisama F, Alisch R, Matthews S, Nakura J, Miki T, Ousais S, et al. Science. 1996;272:258–262. - PubMed
-
- Gray M D, Shen J-C, Kamath-Loeb A S, Blank A, Sopher B L, Martin G M, Oshima J, Loeb L A. Nat Genet. 1997;17:100–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

