Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts
- PMID: 10485653
- PMCID: PMC2789737
- DOI: 10.1016/s1074-7613(00)80093-x
Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts
Abstract
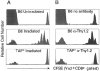
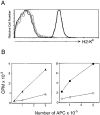
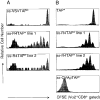
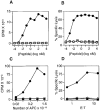
In the absence of thymic emigration, the peripheral T cell pool is maintained by division of mature lymphocytes. We have examined the molecular interactions required for peripheral CD8+ T cell expansion in lymphopenic mice without conventional antigenic stimulation. Expansion of CD8+ T cells in lymphopenic hosts was found to be peptide specific. An antagonist peptide known to serve as a ligand for positive selection of these T cells promoted expansion; however, a control peptide that binds the same class I molecule did not. Surprisingly, the cells undergoing proliferation in lymphopenic hosts did not mature to cytotoxic effectors and displayed a partially activated surface phenotype. These data suggest that division of T cells in the periphery of lymphopenic hosts requires specific recognition of self-peptide/MHC complexes, similar to the signal for thymocyte maturation.
Figures

Similar articles
-
Homeostatic proliferation of a Qa-1b-restricted T cell: a distinction between the ligands required for positive selection and for proliferation in lymphopenic hosts.J Immunol. 2004 Nov 15;173(10):6065-71. doi: 10.4049/jimmunol.173.10.6065. J Immunol. 2004. PMID: 15528342
-
A low affinity TCR ligand restores positive selection of CD8+ T cells in vivo.J Immunol. 2001 Jun 1;166(11):6602-7. doi: 10.4049/jimmunol.166.11.6602. J Immunol. 2001. PMID: 11359813
-
Il-12 enhances CD8 T cell homeostatic expansion.J Immunol. 2001 May 1;166(9):5515-21. doi: 10.4049/jimmunol.166.9.5515. J Immunol. 2001. PMID: 11313390
-
Survival and homeostatic proliferation of naive peripheral CD4+ T cells in the absence of self peptide:MHC complexes.J Immunol. 2000 Sep 1;165(5):2458-64. doi: 10.4049/jimmunol.165.5.2458. J Immunol. 2000. PMID: 10946271
-
Affinity of thymic self-peptides for the TCR determines the selection of CD8(+) T lymphocytes in the thymus.Int Immunol. 2000 Sep;12(9):1353-63. doi: 10.1093/intimm/12.9.1353. Int Immunol. 2000. PMID: 10967031
Cited by
-
Suppression of the Immune Response by Syngeneic Splenocytes Adoptively Transferred to Sublethally Irradiated Mice.Acta Naturae. 2021 Jan-Mar;13(1):116-126. doi: 10.32607/actanaturae.11252. Acta Naturae. 2021. PMID: 33959391 Free PMC article.
-
Expansion of an Unusual Virtual Memory CD8+ Subpopulation Bearing Vα3.2 TCR in Themis-Deficient Mice.Front Immunol. 2021 Apr 7;12:644483. doi: 10.3389/fimmu.2021.644483. eCollection 2021. Front Immunol. 2021. PMID: 33897691 Free PMC article.
-
Pre-T Cell Receptors (Pre-TCRs) Leverage Vβ Complementarity Determining Regions (CDRs) and Hydrophobic Patch in Mechanosensing Thymic Self-ligands.J Biol Chem. 2016 Dec 2;291(49):25292-25305. doi: 10.1074/jbc.M116.752865. Epub 2016 Oct 5. J Biol Chem. 2016. PMID: 27707880 Free PMC article.
-
Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells.J Exp Med. 2002 Jun 17;195(12):1523-32. doi: 10.1084/jem.20020066. J Exp Med. 2002. PMID: 12070280 Free PMC article.
-
Graft-versus-leukemia effects associated with detectable Wilms tumor-1 specific T lymphocytes after allogeneic stem-cell transplantation for acute lymphoblastic leukemia.Blood. 2007 Sep 15;110(6):1924-32. doi: 10.1182/blood-2007-03-076844. Epub 2007 May 15. Blood. 2007. PMID: 17505014 Free PMC article.
References
-
- Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:558–559. - PubMed
-
- Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC, Hogquist KA, Gascoigne NRJ, Travers PJ. Qualitiative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. - PubMed
-
- Allen PM. Peptides in positive and negative selection: a delicate balance. Cell. 1994;76:593–596. - PubMed
-
- Bacik I, Cox JH, Anderson R, Yewdell JW, Bennik JR. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptide is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol. 1994;152:381–387. - PubMed
-
- Barnden MJ, Heath WR, Rodda S, Carbone FR. Peptide antagonists that promote positive selection are inefficient at T cell activation and thymocyte deletion. Eur J Immunol. 1994;24:2452–2456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

