Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication
- PMID: 10482593
- PMCID: PMC112860
- DOI: 10.1128/JVI.73.10.8415-8426.1999
Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication
Abstract
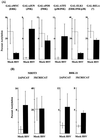
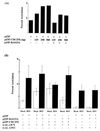
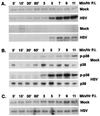
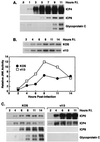
Signal transduction pathways convey signals generated at the cell surface into the cell nucleus in order to initiate a program of gene expression that is characteristic for particular stimuli. Here we present evidence that infection by herpes simplex virus type 1 activated the two terminal kinases, cJUN N-terminal kinase (JNK) and p38, of stress-activated signal transduction kinase cascades. By using a solid-phase kinase assay, a phospho-specific antibody, and extracts prepared from a variety of infected cell types, we determined that activation of both kinases began 3 to 4 h postinfection (p.i.) and remained elevated out to 14 h p.i. Through the use of UV-irradiated or antibody-neutralized wild-type virus and the temperature-sensitive mutant tsB7, the high level of JNK activation was shown to be dependent on viral gene expression. Activation of JNK following infection by vi13, an ICP4 mutant virus that does not express early or late genes, suggested that only virus entry and immediate-early gene expression were necessary for JNK activation. The activation of JNK and p38 correlated with increased chloramphenicol acetyltransferase (CAT) activity in reporter assays dependent upon the activity of cJUN and ATF2 trans-activation domains. Increased CAT activity dependent on TRE and CRE promoter sites was also observed in response to herpes simplex virus infection. The activities of ERK and ERK-dependent transcription factors were unchanged or depressed following infection, showing that activation of JNK and p38 was a specific event. Finally, the activation of JNK was important for the efficiency of viral replication. The yield of virus in NIH 3T3 cells stably expressing JIP-1, an inhibitor of JNK translocation to the nucleus, was reduced 70% compared to that of control cells, in single-step growth experiments.
Figures


Similar articles
-
BX-795 inhibits HSV-1 and HSV-2 replication by blocking the JNK/p38 pathways without interfering with PDK1 activity in host cells.Acta Pharmacol Sin. 2017 Mar;38(3):402-414. doi: 10.1038/aps.2016.160. Epub 2017 Jan 23. Acta Pharmacol Sin. 2017. PMID: 28112176 Free PMC article.
-
Herpes simplex virus ICP27 activation of stress kinases JNK and p38.J Virol. 2005 Jul;79(13):8348-60. doi: 10.1128/JVI.79.13.8348-8360.2005. J Virol. 2005. PMID: 15956580 Free PMC article.
-
Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells.J Virol. 2002 Jun;76(12):5937-48. doi: 10.1128/jvi.76.12.5937-5948.2002. J Virol. 2002. PMID: 12021326 Free PMC article.
-
Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1.J Biol Chem. 1999 Feb 19;274(8):5097-103. doi: 10.1074/jbc.274.8.5097. J Biol Chem. 1999. PMID: 9988758
-
Sounding the alarm: protein kinase cascades activated by stress and inflammation.J Biol Chem. 1996 Oct 4;271(40):24313-6. doi: 10.1074/jbc.271.40.24313. J Biol Chem. 1996. PMID: 8798679 Review. No abstract available.
Cited by
-
β-cantenin is potentially involved in the regulation of c-Jun signaling following bovine herpesvirus 1 infection.Vet Microbiol. 2020 Sep;248:108804. doi: 10.1016/j.vetmic.2020.108804. Epub 2020 Aug 8. Vet Microbiol. 2020. PMID: 32827927 Free PMC article.
-
Transient fasting enhances replication of oncolytic herpes simplex virus in glioblastoma.Am J Cancer Res. 2016 Jan 15;6(2):300-11. eCollection 2016. Am J Cancer Res. 2016. PMID: 27186404 Free PMC article.
-
Posttranslational processing of infected cell proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize.J Virol. 2001 Sep;75(17):7904-12. doi: 10.1128/jvi.75.17.7904-7912.2001. J Virol. 2001. PMID: 11483735 Free PMC article.
-
Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth.J Virol. 2000 Nov;74(22):10417-29. doi: 10.1128/jvi.74.22.10417-10429.2000. J Virol. 2000. PMID: 11044086 Free PMC article.
-
A MicroRNA Derived from Adenovirus Virus-Associated RNAII Promotes Virus Infection via Posttranscriptional Gene Silencing.J Virol. 2019 Jan 4;93(2):e01265-18. doi: 10.1128/JVI.01265-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30355689 Free PMC article.
References
-
- Alvarez E, Northwood I C, Gonzales F A, Latour D A, Seth A, Abate C, Curran T, Davis R J. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. J Biol Chem. 1991;266:15277–15285. - PubMed
-
- Angel P, Karin M. The role of Jun, Fos, and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

