Identification of an internal ribosome entry segment in the 5' region of the mouse VL30 retrotransposon and its use in the development of retroviral vectors
- PMID: 10482590
- PMCID: PMC112857
- DOI: 10.1128/JVI.73.10.8393-8402.1999
Identification of an internal ribosome entry segment in the 5' region of the mouse VL30 retrotransposon and its use in the development of retroviral vectors
Abstract
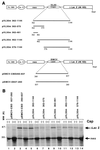
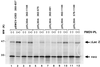
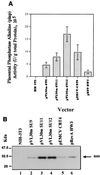
Mouse virus-like 30S RNAs (VL30m) constitute a family of retrotransposons, present at 100 to 200 copies, dispersed in the mouse genome. They display little sequence homology to Moloney murine leukemia virus (MoMLV), do not encode virus-like proteins, and have not been implicated in retroviral carcinogenesis. However, VL30 RNAs are efficiently packaged into MLV particles that are propagated in cell culture. In this study, we addressed whether the 5' region of VL30m could replace the 5' leader of MoMLV functionally in a recombinant vector construct. Our data confirm that the putative packaging sequence of VL30 is located within the 5' region (nucleotides 362 to 1149 with respect to the cap structure) and that it can replace the packaging sequence of MoMLV. We also show that VL30m contains an internal ribosome entry segment (IRES) in the 5' region, as do MoMLV, Friend murine leukemia virus, Harvey murine sarcoma virus, and avian reticuloendotheliosis virus type A. Our data show that both the packaging and IRES functions of the 5' region of VL30m RNA can be efficiently used to develop retrotransposon-based vectors.
Figures


Similar articles
-
Stable MLV-VL30 dicistronic retroviral vectors with a VL30 or MoMLV sequence promoting both packaging of genomic RNA and expression of the 3' cistron.Hum Gene Ther. 1996 Mar 20;7(5):603-12. doi: 10.1089/hum.1996.7.5-603. Hum Gene Ther. 1996. PMID: 8845385
-
An internal ribosomal entry signal in the rat VL30 region of the Harvey murine sarcoma virus leader and its use in dicistronic retroviral vectors.J Virol. 1995 Oct;69(10):6400-7. doi: 10.1128/JVI.69.10.6400-6407.1995. J Virol. 1995. PMID: 7666541 Free PMC article.
-
Characterization of an internal ribosomal entry segment within the 5' leader of avian reticuloendotheliosis virus type A RNA and development of novel MLV-REV-based retroviral vectors.Hum Gene Ther. 1997 Nov 1;8(16):1855-65. doi: 10.1089/hum.1997.8.16-1855. Hum Gene Ther. 1997. PMID: 9382952
-
A small and efficient dimerization/packaging signal of rat VL30 RNA and its use in murine leukemia virus-VL30-derived vectors for gene transfer.J Virol. 1994 Feb;68(2):661-7. doi: 10.1128/JVI.68.2.661-667.1994. J Virol. 1994. PMID: 8289369 Free PMC article.
-
Analytical study of rat retrotransposon VL30 RNA dimerization in vitro and packaging in murine leukemia virus.J Mol Biol. 1994 Jul 29;240(5):434-44. doi: 10.1006/jmbi.1994.1459. J Mol Biol. 1994. PMID: 8046749
Cited by
-
The human immunodeficiency virus type 1 gag gene encodes an internal ribosome entry site.J Virol. 2001 Jan;75(1):181-91. doi: 10.1128/JVI.75.1.181-191.2001. J Virol. 2001. PMID: 11119587 Free PMC article.
-
Translational control of retroviruses.Nat Rev Microbiol. 2007 Feb;5(2):128-40. doi: 10.1038/nrmicro1599. Nat Rev Microbiol. 2007. PMID: 17224922 Free PMC article. Review.
-
Characterization of two distinct RNA domains that regulate translation of the Drosophila gypsy retroelement.RNA. 2004 Mar;10(3):504-15. doi: 10.1261/rna.5185604. RNA. 2004. PMID: 14970395 Free PMC article.
-
Pr77 and L1TcRz: A dual system within the 5'-end of L1Tc retrotransposon, internal promoter and HDV-like ribozyme.Mob Genet Elements. 2012 Jan 1;2(1):1-7. doi: 10.4161/mge.19233. Mob Genet Elements. 2012. PMID: 22754746 Free PMC article.
-
The Mytilus chilensis Steamer-like Element-1 Retrotransposon Antisense mRNA Harbors an Internal Ribosome Entry Site That Is Modulated by hnRNPK.Viruses. 2024 Mar 5;16(3):403. doi: 10.3390/v16030403. Viruses. 2024. PMID: 38543768 Free PMC article.
References
-
- Akiri G, Nahari D, Finkelstein Y, Le S Y, Elroy-Stein O, Levi B Z. Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription. Oncogene. 1998;17:227–236. - PubMed
-
- Attal J, Theron M C, Taboit F, Cajero-Juarez M, Kann G, Bolifraud P, Houdebine L M. The RU5 (′R′) region from human leukaemia viruses (HTLV-1) contains an internal ribosome entry site (IRES)-like sequence. FEBS LETT. 1996;392:220–224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

