The yeast trimeric guanine nucleotide-binding protein alpha subunit, Gpa2p, controls the meiosis-specific kinase Ime2p activity in response to nutrients
- PMID: 10454558
- PMCID: PMC84533
- DOI: 10.1128/MCB.19.9.6110
The yeast trimeric guanine nucleotide-binding protein alpha subunit, Gpa2p, controls the meiosis-specific kinase Ime2p activity in response to nutrients
Abstract
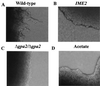
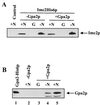
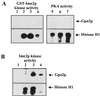
Saccharomyces cerevisiae Gpa2p, the alpha subunit of a heterotrimeric guanine nucleotide-binding protein (G protein), is involved in the regulation of vegetative growth and pseudohyphal development. Here we report that Gpa2p also controls sporulation by interacting with the regulatory domain of Ime2p (Sme1p), a protein kinase essential for entrance of meiosis and sporulation. Protein-protein interactions between Gpa2p and Ime2p depend on the GTP-bound state of Gpa2p and correlate with down-regulation of Ime2p kinase activity in vitro. Overexpression of Ime2p inhibits pseudohyphal development and enables diploid cells to sporulate even in the presence of glucose or nitrogen. In contrast, overexpression of Gpa2p in cells simultaneously overproducing Ime2p results in a drastic reduction of sporulation efficiency, demonstrating an inhibitory effect of Gpa2p on Ime2p function. Furthermore, deletion of GPA2 accelerates sporulation on low-nitrogen medium. These observations are consistent with the following model. In glucose-containing medium, diploid cells do not sporulate because Ime2p is inactive or expressed at low levels. Upon starvation, expression of Gpa2p and Ime2p is induced but sporulation is prevented as long as nitrogen is present in the medium. The negative control of Ime2p kinase activity is exerted at least in part through the activated form of Gpa2p and is released as soon as nutrients are exhausted. This model attributes a switch function to Gpa2p in the meiosis-pseudohyphal growth decision.
Figures


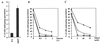


Similar articles
-
Krh1p and Krh2p act downstream of the Gpa2p G(alpha) subunit to negatively regulate haploid invasive growth.J Cell Sci. 2003 Feb 15;116(Pt 4):701-10. doi: 10.1242/jcs.00266. J Cell Sci. 2003. PMID: 12538771
-
Phosphorylation of Ime2 regulates meiotic progression in Saccharomyces cerevisiae.J Biol Chem. 2006 Jul 7;281(27):18307-16. doi: 10.1074/jbc.M602349200. Epub 2006 May 9. J Biol Chem. 2006. PMID: 16684773
-
Glucose inhibits meiotic DNA replication through SCFGrr1p-dependent destruction of Ime2p kinase.Mol Cell Biol. 2005 Jan;25(1):440-50. doi: 10.1128/MCB.25.1.440-450.2005. Mol Cell Biol. 2005. PMID: 15601864 Free PMC article.
-
Ime2p and Cdc28p: co-pilots driving meiotic development.J Cell Biochem. 2004 Aug 1;92(5):1025-33. doi: 10.1002/jcb.20131. J Cell Biochem. 2004. PMID: 15258924 Review.
-
Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae.Mol Microbiol. 1999 Sep;33(5):904-18. doi: 10.1046/j.1365-2958.1999.01538.x. Mol Microbiol. 1999. PMID: 10476026 Review.
Cited by
-
Evolution of Ime2 phosphorylation sites on Cdk1 substrates provides a mechanism to limit the effects of the phosphatase Cdc14 in meiosis.Mol Cell. 2007 Mar 9;25(5):689-702. doi: 10.1016/j.molcel.2007.02.012. Mol Cell. 2007. PMID: 17349956 Free PMC article.
-
Pseudohyphal differentiation defect due to mutations in GPCR and ammonium signaling is suppressed by low glucose concentration: a possible integrated role for carbon and nitrogen limitation.Curr Genet. 2008 Aug;54(2):71-81. doi: 10.1007/s00294-008-0202-1. Epub 2008 Jul 12. Curr Genet. 2008. PMID: 18622617
-
Global phenotype screening and transcript analysis outlines the inhibitory mode(s) of action of two amphibian-derived, alpha-helical, cationic peptides on Saccharomyces cerevisiae.Antimicrob Agents Chemother. 2007 Nov;51(11):3948-59. doi: 10.1128/AAC.01007-07. Epub 2007 Sep 10. Antimicrob Agents Chemother. 2007. PMID: 17846143 Free PMC article.
-
Flo11p adhesin required for meiotic differentiation in Saccharomyces cerevisiae minicolonies grown on plastic surfaces.FEMS Yeast Res. 2011 Mar;11(2):223-32. doi: 10.1111/j.1567-1364.2010.00712.x. Epub 2011 Jan 14. FEMS Yeast Res. 2011. PMID: 21205160 Free PMC article.
-
Glucose induction pathway regulates meiosis in Saccharomyces cerevisiae in part by controlling turnover of Ime2p meiotic kinase.FEMS Yeast Res. 2008 Aug;8(5):676-84. doi: 10.1111/j.1567-1364.2008.00406.x. Epub 2008 Jul 8. FEMS Yeast Res. 2008. PMID: 18616605 Free PMC article.
References
-
- Bardwell L, Cook J G, Inouye C J, Thorner J. Signal propagation and regulation in mating pheromone response pathway of the yeast Saccharomyces cerevisiae. Dev Biol. 1994;166:363–379. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Broach J R. Ras-regulated signaling processes in Saccharomyces cerevisiae. Curr Opin Genet Dev. 1991;1:370–377. - PubMed
-
- Cameron S, Levin L, Zoller M, Wigler M. cAMP-independent control of sporulation, glycogen metabolism, and heat shock resistance in S. cerevisiae. Cell. 1988;53:555–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
