Insoluble gamma-tubulin-containing structures are anchored to the apical network of intermediate filaments in polarized CACO-2 epithelial cells
- PMID: 10444072
- PMCID: PMC2150552
- DOI: 10.1083/jcb.146.3.645
Insoluble gamma-tubulin-containing structures are anchored to the apical network of intermediate filaments in polarized CACO-2 epithelial cells
Abstract
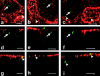
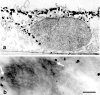
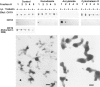
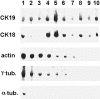
We have previously shown that a thin ( approximately 1 microm) layer of intermediate filaments located beneath the apical membrane of a variety of simple epithelial cells participates in the organization of apical microfilaments and microtubules. Here, I confirmed the apical distribution of gamma-tubulin-containing structures (potential microtubule-organizing centers) in CACO-2 cells and demonstrated perfect colocalization of centrosomes and nearly 50% of noncentrosomal gamma-tubulin with apical intermediate filaments, but not with apical F-actin. Furthermore, the antisense-oligonucleotide-mediated downregulation of cytokeratin 19, using two different antisense sequences, was more efficient than anticytoskeletal agents to delocalize centrosomes. Electron microscopy colocalization suggests that binding occurs at the outer boundary of the pericentriolar material. Type I cytokeratins 18 and 19 present in these cells specifically coimmunoprecipitated in multi-protein fragments of the cytoskeleton with gamma-tubulin. The size and shape of the fragments, visualized at the EM level, indicate that physical trapping is an unlikely explanation for this result. Drastic changes in the extraction protocol did not affect coimmunoprecipitation. These results from three independent techniques, indicate that insoluble gamma-tubulin-containing structures are attached to apical intermediate filaments.
Figures
Similar articles
-
GCP6 binds to intermediate filaments: a novel function of keratins in the organization of microtubules in epithelial cells.Mol Biol Cell. 2007 Mar;18(3):781-94. doi: 10.1091/mbc.e06-03-0201. Epub 2006 Dec 20. Mol Biol Cell. 2007. PMID: 17182859 Free PMC article.
-
The apical submembrane cytoskeleton participates in the organization of the apical pole in epithelial cells.J Cell Biol. 1997 Apr 21;137(2):359-75. doi: 10.1083/jcb.137.2.359. J Cell Biol. 1997. PMID: 9128248 Free PMC article.
-
Polarity and nucleation of microtubules in polarized epithelial cells.Cell Motil Cytoskeleton. 1995;32(4):273-88. doi: 10.1002/cm.970320404. Cell Motil Cytoskeleton. 1995. PMID: 8608606
-
Intermediate filaments: a role in epithelial polarity.Exp Cell Res. 2007 Jun 10;313(10):2255-64. doi: 10.1016/j.yexcr.2007.02.030. Epub 2007 Mar 12. Exp Cell Res. 2007. PMID: 17425955 Free PMC article. Review.
-
The experimental manipulation of keratin expression and organization in epithelial cells and somatic cell hybrids.Curr Top Dev Biol. 1987;22:69-96. doi: 10.1016/s0070-2153(08)60099-x. Curr Top Dev Biol. 1987. PMID: 2443317 Review. No abstract available.
Cited by
-
Cytoskeletal actin microfilaments and the transient outward potassium current in hypertrophied rat ventriculocytes.J Physiol. 2002 Jun 1;541(Pt 2):411-21. doi: 10.1113/jphysiol.2002.019562. J Physiol. 2002. PMID: 12042348 Free PMC article.
-
AMPK-dependent phosphorylation of cingulin reversibly regulates its binding to actin filaments and microtubules.Sci Rep. 2018 Oct 19;8(1):15550. doi: 10.1038/s41598-018-33418-7. Sci Rep. 2018. PMID: 30341325 Free PMC article.
-
Genetically induced microtubule disruption in the mouse intestine impairs intracellular organization and transport.Mol Biol Cell. 2018 Jul 1;29(13):1533-1541. doi: 10.1091/mbc.E18-01-0057. Epub 2018 May 9. Mol Biol Cell. 2018. PMID: 29742015 Free PMC article.
-
MCC is a centrosomal protein that relocalizes to non-centrosomal apical sites during intestinal cell differentiation.J Cell Sci. 2022 Nov 1;135(21):jcs259272. doi: 10.1242/jcs.259272. Epub 2022 Oct 28. J Cell Sci. 2022. PMID: 36217793 Free PMC article.
-
Adult human CD133/1(+) kidney cells isolated from papilla integrate into developing kidney tubules.Biochim Biophys Acta. 2011 Oct;1812(10):1344-57. doi: 10.1016/j.bbadis.2011.01.010. Epub 2011 Jan 19. Biochim Biophys Acta. 2011. PMID: 21255643 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

